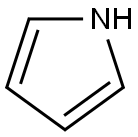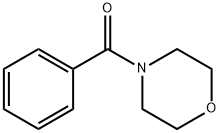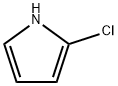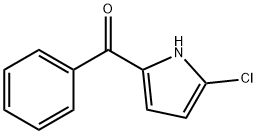
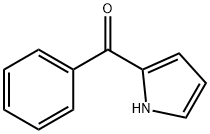
(5-chloro-1H-pyrrol-2-yl)-phenylmethanone synthesis
- Product Name:(5-chloro-1H-pyrrol-2-yl)-phenylmethanone
- CAS Number:142231-06-9
- Molecular formula:C11H8ClNO
- Molecular Weight:205.64
7697-46-3
159 suppliers
$79.00/250mg

142231-06-9
20 suppliers
inquiry
Yield:142231-06-9 50.6%
Reaction Conditions:
with copper dichloride in acetonitrile; for 48 h;Reflux;
Steps:
2.10 Compound 8
To 200 mL of acetonitrile was added compound 7 (17 g, 0.083 mol) and 25 g of CuCl2, then the reaction mixture was heated to reflux with stirring for 48 h. After the reaction was cooled to room temperature, H2SO4 (10%, 101 mL) was added into reaction mixture, and the reaction keep stirring for 70 min. The resulting mixture was extracted by ethyl acetate (30 mL×3). The organic phase was washed by water (30 mL×3), then dried over Na2SO4 and evaporated in vacuum. The residue was purified by silica gel column (hexane:ethyl actate:DCM = 200: 1: 20) to afford 8 (8.6 g, 50.6%). 1H NMR (400MHz, CDCl3), δ 10.95 (s, 1H), 7.95 (d, 2H), 7.66 (t, 1H), 7.55 (t, 2H), 7.29 (s, 1H), 6.38 (s, 1H).
References:
Chen, Ji-An;Zhang, Zhen-Yu;Gao, Jie;Tan, Jia-Hui;Gu, Xian-Feng [Tetrahedron Letters,2019,vol. 60,# 18,p. 1226 - 1230] Location in patent:supporting information

7697-46-3
159 suppliers
$79.00/250mg

142231-06-9
20 suppliers
inquiry
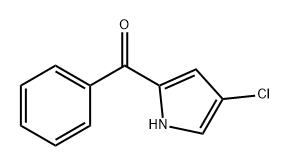
142231-07-0
0 suppliers
inquiry
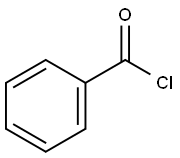
98-88-4
583 suppliers
$10.00/5g

142231-06-9
20 suppliers
inquiry