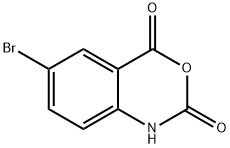
5-Bromoisatoic anhydride synthesis
- Product Name:5-Bromoisatoic anhydride
- CAS Number:4692-98-2
- Molecular formula:C8H4BrNO3
- Molecular Weight:242.03
1HNMR(DMSO-d6): ! 11.85 (s, br., 1H), 7.96 (dd, J = 0.8, 8.7 Hz, 1H), 7.87 (dd, J = 2.3, 8.7 Hz, 1H), 7.09 (dd, J = 8.8, 0.8 Hz, 1H).
32315-10-9
417 suppliers
$10.00/1g
5794-88-7
466 suppliers
$6.00/10g

4692-98-2
222 suppliers
$13.00/1g
Yield:4692-98-2 98%
Reaction Conditions:
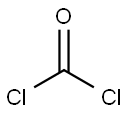
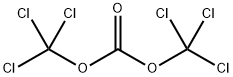
with pyridine in dichloromethane;acetonitrile at 50 - 55; for 2 h;
Steps:
2.a
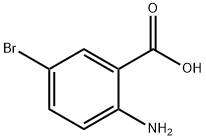
a) Preparation of 6-bromo-1H-benzo[d][1,3]oxazine-2,4-dione A solution of 2-amino-5-bromo-benzoic acid (280 g, 1.3 mol) in acetonitrile (1.3 l) was warmed up to 50-55° C. Pyridine (206 g, 2.61 mol) and a solution of triphosgene (128.8 g, 0.43 mol) in dichloromethane (720 ml) were simultaneously added dropwise. After completion of the addition, the mixture was stirred at 50-55° C. for an additional 2 h. The solvent was removed under reduced pressure and water was added. The precipitate was collected by filtration, successively washed with water and chilled dichloromethane, and then dried under vacuum to afford the desired product 6-bromo-1H-benzo[d][1,3]oxazine-2,4-dione (304 g, 98%). This material was used in the next step without further purification.
References:
Chen, Li;Chen, Shaoqing;Michoud, Christophe US2006/63804, 2006, A1 Location in patent:Page/Page column 11
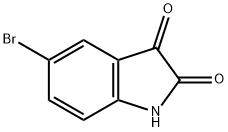
87-48-9
288 suppliers
$10.00/5g

4692-98-2
222 suppliers
$13.00/1g
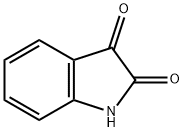
91-56-5
457 suppliers
$5.00/25g

4692-98-2
222 suppliers
$13.00/1g
124-38-9
129 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry
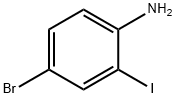
66416-72-6
189 suppliers
$6.00/250mg

4692-98-2
222 suppliers
$13.00/1g