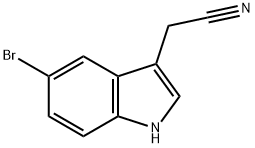
5-Bromoindole-3-acetonitrile synthesis
- Product Name:5-Bromoindole-3-acetonitrile
- CAS Number:774-14-1
- Molecular formula:C10H7BrN2
- Molecular Weight:235.08
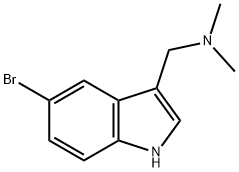
830-93-3
58 suppliers
$60.00/500mg
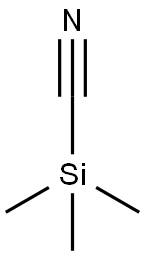
7677-24-9
394 suppliers
$19.00/5g

74-88-4
351 suppliers
$15.00/10g

774-14-1
34 suppliers
inquiry
Yield:774-14-1 51%
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuran;hexane;water;ethyl acetate;benzene;
Steps:
63.2 (5-Bromo-1H-indol-3-yl)-acetonitrile
Step 2 (5-Bromo-1H-indol-3-yl)-acetonitrile To an ice cooled suspension of (5-bromo-1H-indol-3-ylmethyl)-dimethyl-amine (11.2 g, 44.1 mmol) in benzene (145 mL) was added iodomethane (8.2 mL, 130 mmol). The reaction mixture was stirred overnight at room temperature and concentrated. The residue was dissolved in THF (210 mL). Trimethylsilyl cyanide (11.7 mL, 87.7 mmol) and tetrabutylammonium fluoride (140 mL, 140 mmol of a 1.0 M solution in THF) were added. After stirring for 2.5 hours, at room temperature, water (12 mL) was added. The mixture was partially concentrated. The residue was partitioned in ethyl acetate and water. The organic phase was washed with water and brine, dried over anhydrous magnesium sulfate and evaporated to dryness. Purification by flash chromatography using 15-35% ethyl acetate in hexane as an eluant afforded (5-bromo-1H-indol-3-yl)-acetonitrile (5.30 g, 51%) as a mauve solid, mp 105-106° C. 1H-NMR (300 MHz, DMSO-d6): δ11.35 (s, 1H), 7.8 (d, 1H, J=2.2 Hz), 7.35-7.45 (m, 2H), 7.2-7.3 (m, 1H), and 4.0 ppm (s, 2H).
References:
US2003/125371,2003,A1
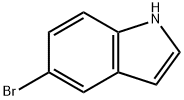
10075-50-0
691 suppliers
$5.00/10g

774-14-1
34 suppliers
inquiry
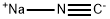
773837-37-9
0 suppliers
inquiry

10075-50-0
691 suppliers
$5.00/10g

74-88-4
351 suppliers
$15.00/10g

774-14-1
34 suppliers
inquiry

830-93-3
58 suppliers
$60.00/500mg

774-14-1
34 suppliers
inquiry