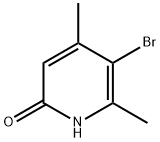
5-broMo-4,6-diMethylpyridin-2-ol synthesis
- Product Name:5-broMo-4,6-diMethylpyridin-2-ol
- CAS Number:89694-55-3
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
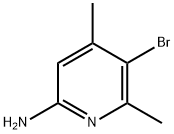
89856-44-0
113 suppliers
$45.00/500mg

89694-55-3
17 suppliers
inquiry
Yield:89694-55-3 58%
Reaction Conditions:
Stage #1: 2-amino-5-bromo-4,6-dimethylpyridinewith hydrogenchloride;waterReflux;
Stage #2: with sodium nitrite in water at 20;
Steps:
Intermediate 60{(S)-6-[(R)-4-(1 ,2,4-Trimethyl-6-oxo-1 ,6-dihvdro-pyridin-3-yl)-indan-1 -yloxyl-2,3- dihydro-benzofuran-3-yl)-acetic acid methyl esterStep 1 : 5-bromo-4,6-dimethyl-pyridin-2-ol5-Bromo-4,6-dimethyl-pyridin-2-ylamine (2.01 g) in water (120 mL) and 32% aqueous HCI (40 mL) is heated to reflux temperature and the solution is filtered hot. NaN02 (2.00 g) dissolved in water (40 mL) is added to the hot filtrate with vigorous stirring. The resulting mixture is stirred at room temperature overnight. The mixture is cooled in an ice bath and the precipitate is separated by filtration. The precipitate is washed with water and dried to give the title compound. Yield: 1 .17 g (58% of theory); LC (method 1 ): tR = 0.82 min; Mass spectrum (EST): m/z = 202/204 (Br) [M+H]+.
References:
WO2012/72691,2012,A1 Location in patent:Page/Page column 100
23819-87-6
61 suppliers
$21.00/1g

89694-55-3
17 suppliers
inquiry

5407-88-5
3 suppliers
inquiry

89694-55-3
17 suppliers
inquiry
99179-94-9
1 suppliers
inquiry

89694-55-3
17 suppliers
inquiry
5407-87-4
206 suppliers
$9.00/1g

89694-55-3
17 suppliers
inquiry