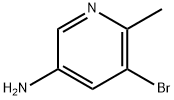
5-AMINO-3-BROMO-2-METHYLPYRIDINE synthesis
- Product Name:5-AMINO-3-BROMO-2-METHYLPYRIDINE
- CAS Number:186593-43-1
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
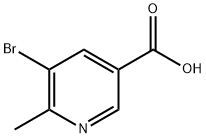
1190862-72-6

186593-43-1
The general procedure for the synthesis of 2-methyl-3-bromo-5-aminopyridine from 5-bromo-6-methylnicotinic acid was as follows: 5-bromo-6-methylpyridine-3-carboxylic acid (1 g, 4.63 mmol) was dissolved in a solvent mixture of tert-butanol (6.6 mL) and N,N-dimethylformamide (15 mL), followed by triethylamine (5.15 mL, 37.04 mmol) and diphenylphosphoryl azide (0.51 mL, 5.093 mmol). The reaction mixture was heated to 100°C and stirred for 2 hours. Upon completion of the reaction, the progress was monitored by thin layer chromatography (TLC). The solvent was evaporated to dryness and the residue was dissolved in saturated sodium bicarbonate solution and extracted with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate and filtered and the solvent was evaporated to dryness. The residue was purified by silica gel column chromatography to give a white solid. The white solid was dissolved in dichloromethane (10 mL), a dioxane solution of 4N HCl was added and stirred for 2 hours at room temperature. Upon completion of the reaction, the solvent was evaporated to dryness to give 5-bromo-6-methylpyridin-3-ylamine (white solid, 130 mg).

1190862-72-6
60 suppliers
$40.00/100mg

186593-43-1
140 suppliers
$14.00/250mg
Yield:186593-43-1 130 mg
Reaction Conditions:
Stage #1:5-bromo-6-methylnicotinic acid with diphenyl phosphoryl azide;triethylamine in N,N-dimethyl-formamide;tert-butyl alcohol at 100; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane;dichloromethane at 20; for 2 h;
Steps:
19.1 Step: 5-Bromo-6-methyl - pyridin-3-yl-amine
5-Bromo-6-methyl - pyridine-3-carboxylic acid (1 g, 4.63 mmol) wasdissolved in tert-butanol (6.6 mL) and N, N- dimethylformamide (15 mL), andthereto were added triethylamine (5.15 mL, 37.04mmol) and diphenylphosphorylazide (0.51 mL, 5.093 mmol). This mixture was heated to 100° Creaction 2h, after the end of the reaction by TLC, the solvent was evaporatedto dryness, and the residue was dissolved in saturated sodium bicarbonatesolution, extracted with ethyl acetate, dried over anhydrous sodium sulfate,filtered and the solvent was evaporated to dryness, the residue on silica gelcolumn chromatography to give a white solid. The above white solid wasdissolved in dichloromethane (10 mL), was added 4N hydrogen chloride in dioxane, stirred for 2 hours at room temperature. After completion of the reaction, the solvent was evaporated to dryness to give 5-bromo-6-methyl -pyridin-3-ylamine (white solid, 130 mg).
References:
Shanghai Pharmaceuticals Holding Co.,Ltd .;WAN, HUIXIN;LI, CHUNLI;SHI, Chen;Liu, Haiyan;Li, Ping;XIA, Guangxin;HAN, Yanan CN103420977, 2016, B Location in patent:Paragraph 0293-0296
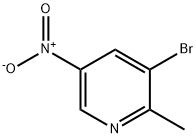
186593-42-0
139 suppliers
$11.00/1g

186593-43-1
140 suppliers
$14.00/250mg

186593-42-0
139 suppliers
$11.00/1g
7439-89-6
460 suppliers
$10.00/25g

186593-43-1
140 suppliers
$14.00/250mg
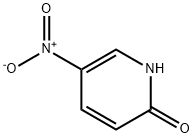
5418-51-9
450 suppliers
$12.72/1gm:

186593-43-1
140 suppliers
$14.00/250mg
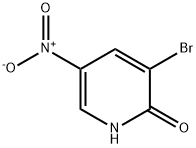
15862-33-6
161 suppliers
$5.00/250mg

186593-43-1
140 suppliers
$14.00/250mg