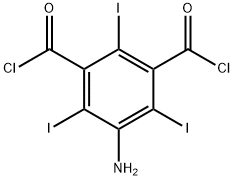
5-Amino-2,4,6-triiodoisophthaloyl dichloride synthesis
- Product Name:5-Amino-2,4,6-triiodoisophthaloyl dichloride
- CAS Number:37441-29-5
- Molecular formula:C8H2Cl2I3NO2
- Molecular Weight:595.73
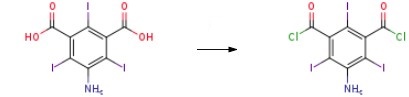
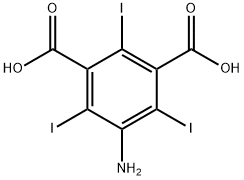
35453-19-1
333 suppliers
$6.00/5g

37441-29-5
259 suppliers
$14.00/1g
Yield:37441-29-5 100%
Reaction Conditions:
with pyridine;thionyl chloride in 1,2-dichloro-ethane at 70 - 85; for 8.5 h;
Steps:
A; 8.a
Preparation (A); Synthesis of 5-Amino-2,4,6-triiodo-isophthaloyl dichloride (1); 5-Amino-2,4,6-triiodo-isophtalic acid (30 g, 0.054 mol) (commercially available from Aldrich), thionyl chloride (8.2 ml, 0.113 mol) and pyridine (0.2 ml) in 1,2 dichloroethane (20 ml) were heated to 70 0C. A portion of thionyl chloride (15.2 ml, 0.21 mol) was added dropwise during VA o 2 hrs, and the mixture was heated to 85 0C for 6 hrs. After cooling the reaction mixture to room temperature, it was poured into 30Og of ice-water. The yellow precipitate that formed was filtered off, sucked dry and then washed with water until washings showed a pH of ca 5. The filter cake was then dried in a vacuum oven at 500C for 3 hrs. A light yellow powder was obtained 31 g (~ quant.) as the desired product.13C NMR (DMSOcZ6) 66, 78.4, 148.9, 149.2, 169MS (ES-) found 593.5 [M-H+], expected 593.7; Example 8; N,N',N"-Tris-[(3(N-2,3-dihydroxypropylcarbamoyl)-2,4,6-triiodo-1-hydroxyl acetamide) phenyl] carbamoyl methyl ethane; Starting material and all other materials were commercially available from Aldrich. a) 5-amino-2,4,6-triiodo-isophtaloyl chloride; 5-Amino-2,4,6-triiodo-isophtalic acid (30 g, 0.054 mol), thionyl chloride (8.2 ml, 0.113 mol) and pyridine (0.2 ml) in 1,2 dichloroethane (20 ml) were heated to 70 0C. A portion of thionyl chloride (15.2 ml, 0.21 mol) was added dropwise during VA to 2 hrs, and the mixture was heated to 85 0C for 6 hrs. After cooling the reaction mixture to room temperature, it was poured into 30Og of ice-water. The yellow precipitate that formed was filtered off, sucked dry and then washed with water until washings showed a pH of ca 5. The filter cake was then dried in a vacuum oven at 50°C for 3 hrs. A light yellow powder was obtained 31 g (~ quant.) as the desired product.13C NMR (DMSOd6) 66, 78.4, 148.9, 149.2, 169MS (ES-) found 593.5 [M-H+], expected 593.7FT-IR (cm"1) 3471, 3372 (NH), 1777 (C=O).
References:
GE HEALTHCARE AS WO2007/94683, 2007, A1 Location in patent:Page/Page column 17; 48-49

35453-19-1
333 suppliers
$6.00/5g
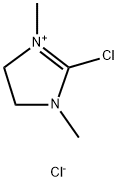
37091-73-9
202 suppliers
$7.00/1g

37441-29-5
259 suppliers
$14.00/1g
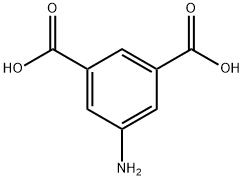
99-31-0
437 suppliers
$6.00/10g

37441-29-5
259 suppliers
$14.00/1g
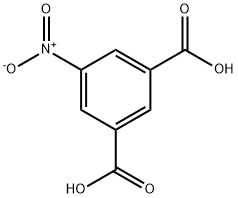
618-88-2
487 suppliers
$13.00/25g

37441-29-5
259 suppliers
$14.00/1g