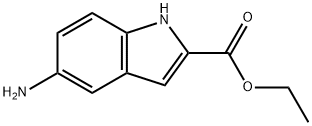
5-amino-1H-Indole-2-carboxylic acid ethyl ester synthesis
- Product Name:5-amino-1H-Indole-2-carboxylic acid ethyl ester
- CAS Number:71086-99-2
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
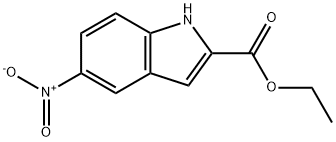
16732-57-3
213 suppliers
$7.00/250mg

71086-99-2
90 suppliers
$65.00/250mg
Yield: 100%
Reaction Conditions:
with ammonium formate;5%-palladium/activated carbon in ethanol for 0.5 h;Heating / reflux;
Steps:
7.o.i
(o) 5-[(5-Phenyl-furan-2-carbonyl)-amino]-lH-indole-2- carboxylic acid (59); (i) 5-Amino-lH-indole-2-carboxylic acid ethyl ester (58) 5-Nitro-lH-indole-2-carboxylic acid ethyl ester (500 mg, 2.1 mmol) was reduced using Method I to give the title compound. Yield: 433 mg, 100%; LC-MS tr 0.84 min; MS (ES+) m/z 205 (M+H); I); The nitro ester was dissolved in EtOH (6 vol) and NH4COOH (4 eq) was added as a solid. 5% Palladium on carbon (10% by weight) was then added under N2 and the resulting mixture heated to reflux under N2 for 30 min. The reaction mixture was filtered through celite and the celite was washed with EtOH (20 vol). The solvent was removed in vacuo to give the product.
References:
PHARMAGENE LABORATORIES LIMITED WO2005/80367, 2005, A1 Location in patent:Page/Page column 67-68; 113-114
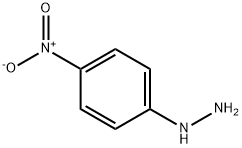
100-16-3
4 suppliers
$45.00/1g

71086-99-2
90 suppliers
$65.00/250mg
![ethyl (2E)-2-[(4-nitrophenyl)hydrazinylidene]propanoate](/CAS/GIF/73647-04-8.gif)
73647-04-8
14 suppliers
inquiry

71086-99-2
90 suppliers
$65.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

16732-57-3
213 suppliers
$7.00/250mg

71086-99-2
90 suppliers
$65.00/250mg
![ethyl (2Z)-2-[(4-nitrophenyl)hydrazinylidene]propanoate](/CAS/20180723/GIF/63190-15-8.gif)
63190-15-8
3 suppliers
inquiry

71086-99-2
90 suppliers
$65.00/250mg