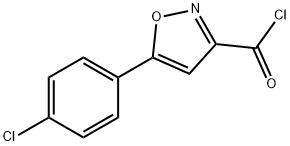
5-(4-CHLORO-PHENYL)-ISOXAZOLE-3-CARBONYL CHLORIDE synthesis
- Product Name:5-(4-CHLORO-PHENYL)-ISOXAZOLE-3-CARBONYL CHLORIDE
- CAS Number:50872-45-2
- Molecular formula:C10H5Cl2NO2
- Molecular Weight:242.06
33282-22-3
71 suppliers
$45.00/100mg

50872-45-2
15 suppliers
$318.00/1g
Yield:-
Reaction Conditions:
with oxalyl dichloride in dichloromethane;N,N-dimethyl-formamide; for 2 h;
Steps:
8
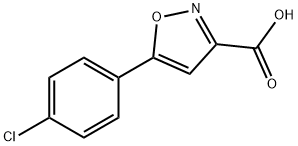
5-(4-Chlorophenyl)isoxazole-3-carboxylic acid (0.120 g, .5 mmol) was allowed to stir in 15 mL Of CH2Cl2. Oxalyl chloride (.192ml, 1.5mmol) was added and the reaction was allowed to stir. Two drops of DMF were added via pipet and the effervescing reaction mixture was allowed to stir two additional hours. The reaction mixture was concentrated to dryness and residual oxalyl chloride was azeotroped using CH2Cl2 (3 x 20 ml). The resulting light-yellow solid was allowed to dry under vacuum for 4 hours. The solid was dissolved in 10 ml of CH2CI2, Intermediate E (0.100 g, 0.5 mmol) was added, and the reaction allowed to stir for 16 hours. LC/MS monitoring indicated reaction completion. The reaction was diluted with 20 mL of CH2CI2, washed with brine (3 x 20 mL) and subsequently dried over MgSO4. Concentration to dryness yielded an off-white solid. Purification via Siθ2 chromatography using a (9/0.9/0.1) mixture Of (CH2CyCH3OHZNH3OH) afforded 34.2 mgs (20% yield) of the title compound as a white solid. 1H NMR (300 MHz, CDCl3) δ 7.72 (m, 2H), 7.45 (m, 2H), 7.25 (m, IH), 6.85 (m, 4H), 4.80 (d, 2H), 4.30 (m, IH), 3.10 (d, 3H), 2.68 (m, 2H), 2.30 (m, 5H), 1.98 (m, 2H), 1.83 (m, 2H).
References:
WO2007/78251,2007,A1 Location in patent:Page/Page column 41-42