6-METHYL-2,4,6-TRIS(TRIFLUOROMETHYL)TETRAHYDROPYRAN-2,4-DIOL synthesis
- Product Name:6-METHYL-2,4,6-TRIS(TRIFLUOROMETHYL)TETRAHYDROPYRAN-2,4-DIOL
- CAS Number:429-01-6
- Molecular formula:C9H9F9O3
- Molecular Weight:336.15
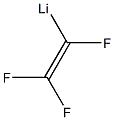
421-50-1
210 suppliers
$15.00/5g

429-01-6
16 suppliers
$45.00/50mg
Yield:429-01-6 73%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran at 20; for 4 h;
Steps:
E 1 preparation of 4,6-dihydroxy-2-methyl-2,4,6-tris(trifluoromethyl)-tetrahydropyran
n a 500 ML three-neck round bottom flask equipped with a teflon stirrer, a thermocouple and a nitrogen inlet, 88 ML of a 1 M THF solution of potassium tert-butoxide were cooled to a temperature ranging from -30° to -50°C. A 40 ML quantity of trifluoroacetone was slowly added to the flask to form a reaction mixture and the reaction mixture was allowed to warm up to room temperature.. After four hours, the reaction mixture was poured onto a dilute solution of hydrochloric acid and the aqueous layer was extracted with ethyl ether.. The combined organic layers were dried and filtered and the solvent was evaporated to provide 36 grams of a white solid product after recrystallization from a hexane solvent.. The percentage yield of the product was 73%.. An analysis of the product identified it as 4,6-dihydroxy-2-methyl-2,4,6-tris(trifluoromethyl)-tetrahydropyran (four stereoisomers, one major).. The mass spectrum and NMR results for the product were as follows: mass spectrum m/z (267, M - CF3), 1H NMR of the major component (CD3OD, ppm) 4.86 (s, 2H), 1.79 (s, 3H), 1.84 - 186 (d, 1 H), 1.91 -1.94 (d, 1 H), 1.97 -2.00 (d, 1 H), 2.1 - 2.17 (d, 1 H); 19F NMR of the major component (CD3OD, ppm) -89.2 (s, CF3), -87.3 (s, CF3), -87.1 (s, CF3).
References:
EP1449839,2004,A2 Location in patent:Page 9

421-50-1
210 suppliers
$15.00/5g
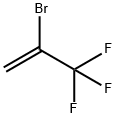
1514-82-5
261 suppliers
$45.00/5G

429-01-6
16 suppliers
$45.00/50mg