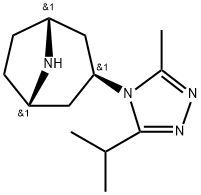
(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane synthesis
- Product Name:(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane
- CAS Number:423165-07-5
- Molecular formula:C13H22N4
- Molecular Weight:234.35
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane (1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/NewsImg/2023-10-31/6383436551081556864474832.jpg)
![8-Benzyl-3-exo-(5-isopropyl-3-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-13-3.gif)
423165-13-3
64 suppliers
$65.00/100 mg
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
113 suppliers
$60.00/100mg
Yield: 100%
Reaction Conditions:
with hydrogen;palladium hydroxide on carbon in ethanol at 20; for 18 h;
Steps:
1; 3
[109] Synthesis of 3-(3-isopropyl-5-methyl-4H-1.2.4-triazol-4-yl)-8- azabicvclor3.2.11octane 103 (Y2a = Y2b = Y2c = H). To a solution of the triazole 102 (0.28 g, 0.86 mmol) in EtOH (9 mL) at ambient temperature was added 20 wt % Pd(OH)2/C (140 mg). The mixture was purged with argon then placed under an H2 atmosphere and stirred at ambient temperature for 18h. The reaction mixture was filtered through a pad of celite, the pad washed with EtOH, and the filtrate was concentrated in vacuo to yield 103 (0.20 g, -100%).; The triazole 102 in EtOH was treated with Pd(OH)2/C (140 mg) under an H2 atmosphere to yield amine 103.
References:
CONCERT PHARMACEUTICALS INC. WO2008/63600, 2008, A2 Location in patent:Page/Page column 11-12; 28
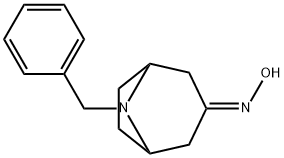
76272-34-9
51 suppliers
$140.00/50 mg
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
113 suppliers
$60.00/100mg
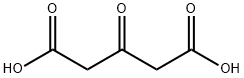
542-05-2
489 suppliers
$6.00/10g
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
113 suppliers
$60.00/100mg
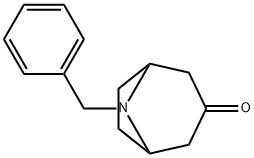
28957-72-4
216 suppliers
$27.00/1g
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
113 suppliers
$60.00/100mg
![3-AMINO-8-BENZYL-8-AZABICYCLO[3.2.1]OCTANE (3-EXO)-](/CAS/GIF/76272-36-1.gif)
76272-36-1
83 suppliers
$42.40/1mg:
![(1R,3s,5S)-3-(3-Isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octane](/CAS/GIF/423165-07-5.gif)
423165-07-5
113 suppliers
$60.00/100mg