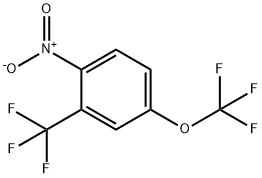
1-NITRO-4-TRIFLUOROMETHOXY-2-TRIFLUOROMETHYL-BENZENE synthesis
- Product Name:1-NITRO-4-TRIFLUOROMETHOXY-2-TRIFLUOROMETHYL-BENZENE
- CAS Number:409114-47-2
- Molecular formula:C8H3F6NO3
- Molecular Weight:275.1

409114-47-2
22 suppliers
inquiry
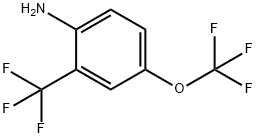
409114-48-3
26 suppliers
$497.07/5MG
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; for 1 h;Inert atmosphere;
Steps:
13
1-Nitro-4-trifluoromethoxy-2-trifluoromethyl-benzene (0.95 g) was dissolved in methanol (90 mL), and the solution was sparged with Ar. Pd/C (10 %, ca. 50 mg) was added, and a balloon filled with hydrogen gas was added. The reaction was stirred for 1 h at RT, and was then filtered through celite. The solvents were removed at 80 mm Hg with heating at or below 35 0C. The product was distilled at 80 mm Hg bulb-to-bulb to give 4-trifluoromethoxy-2-trifluoromethyl- phenylamine as a colorless liquid (0.63 g). The 4-trifluoromethoxy-2-trifluoromethyl-phenylamine (607 mg, 2.5 mmol) was suspended in water (2.6 mL), and cone, sulfuric acid (1 mL) was added. The mixture was heated to boiling and rapidly stirred, and then allowed to cool to RT with continued stirring. The resulting suspension was stirred in an ice bath as sodium nitrite solution (190 mg in 1.2 mL of water) was added drop-wise. After stirring for 20 min in the ice bath, a solution of potassium iodide (0.83 g in 1.0 mL of water) was added in a steady stream. A trace of copper bronze was added and the reaction was briefly heated to boiling. After 1 h at room temperature, the product was extracted into ether and washed with sodium bisulfite and brine, dried over sodium sulfate, and concentrated onto celite. The l-iodo-4-trifluoromethoxy-2-trifluoromethyl-benzene was isolated via silica gel flash chromatography using EtOAc in hexanes (0-10%) to give a yellow oil (560 mg). The l-iodo-4-trifluoromethoxy-2-trifluoromethyl-benzene (530 mg, 1.5 mmol, in 1.0 mL of dry ether) was added drop-wise via syringe to a solution of dry ether (1.5 mL) and nBuLi (0.65 mL, 2.5 M, 1.6 mmol) under Ar and in a dry ice-acetone bath. After 5 min, DMF (0.13 mL, 1.7 mmol) was added, and the reaction was allowed to warm to RT over 30 min. The reaction was quenched with sulfuric acid (5 mL, IN) for 5 min, and the layers were then separated. The organic layer was washed with water and brine, dried over sodium sulfate, and concentrated onto celite. The 4-trifluoromethoxy-2- trifluoromethyl-benzaldehyde was isolated via silica gel flash chromatography using EtOAc in hexanes (0-5%) as a yellow liquid (186 mg).
References:
WO2010/81149,2010,A1 Location in patent:Page/Page column 26-27

409114-47-2
22 suppliers
inquiry
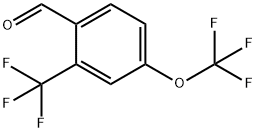
791848-28-7
0 suppliers
inquiry

409114-47-2
22 suppliers
inquiry
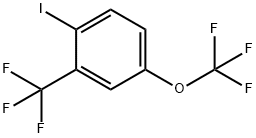
1234790-88-5
0 suppliers
inquiry