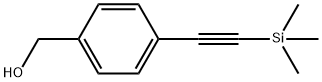
4-(TRIMETHYLSILYLETHYNYL)BENZYL ALCOHOL synthesis
- Product Name:4-(TRIMETHYLSILYLETHYNYL)BENZYL ALCOHOL
- CAS Number:275386-60-2
- Molecular formula:C12H16OSi
- Molecular Weight:204.34
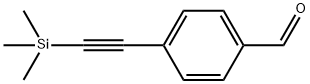
77123-57-0
113 suppliers
$15.00/1g

275386-60-2
70 suppliers
$10.00/100mg
Yield: 94%
Reaction Conditions:
Stage #1:4-[(trimethylsilyl)ethynyl]bezaldehyde with sodium tetrahydroborate in ethanol at 20;
Stage #2: with water;ammonium chloride in ethanol
Steps:
4.B
Part B: Synthesis of 4- [(TrimethylsilvDethynyl-phenyll methanolTo a stirred solution of 4-[(trimethylsilyl)ethynyl]benzaldehyde (0.50 g, 2.47 mmol) in dry ethanol (10 ml) was added sodium borohydride (0.32 g, 8.50 mmol). The reduction was almost instantaneous at room temperature. The reaction mixture was carefully quenched with half-saturated aqueous ammonium chloride solution (30 ml) and extracted with dichloromethane (2 x 20 ml). The combined organic layers were washed with saturated
References:
COMMONWEALTH SCIENTIFIC AND INDUSTRIAL RESEARCH ORGANISATION WO2008/31157, 2008, A1 Location in patent:Page/Page column 64-65
18282-51-4
216 suppliers
$10.00/1g
1066-54-2
469 suppliers
$9.00/10g

275386-60-2
70 suppliers
$10.00/100mg
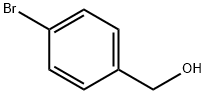
873-75-6
311 suppliers
$6.00/5g

1066-54-2
469 suppliers
$9.00/10g

275386-60-2
70 suppliers
$10.00/100mg
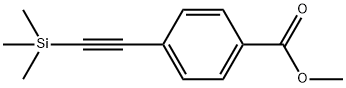
75867-41-3
30 suppliers
$414.00/0.25G

275386-60-2
70 suppliers
$10.00/100mg
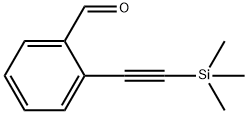
77123-58-1
51 suppliers
$99.50/5g

77123-57-0
113 suppliers
$15.00/1g
![{2-[2-(trimethylsilyl)ethynyl]phenyl}methanol](/CAS/20200401/GIF/229027-92-3.gif)
229027-92-3
1 suppliers
inquiry

275386-60-2
70 suppliers
$10.00/100mg