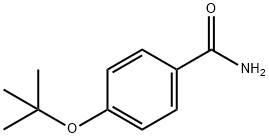
4-TERT-BUTOXYBENZAMIDE synthesis
- Product Name:4-TERT-BUTOXYBENZAMIDE
- CAS Number:99985-67-8
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
1194-02-1
515 suppliers
$6.00/10g
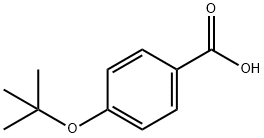
13205-47-5
75 suppliers
$21.00/250mg

99985-67-8
22 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in sodium hydroxide;ethanol;water;dimethyl sulfoxide;
Steps:
7 4-(1,1-dimethylethoxy)benzoic acid
EXAMPLE 7 4-(1,1-dimethylethoxy)benzoic acid A suspension of hexane washed sodium hydride (1.3 grams of a 60% suspension in oil) in 15 ml. of dry dimethylsulfoxide is heated at 70° C. under nitrogen for 1 hour. It is then cooled to 30° C. and 1.5 grams of tertiary butanol is slowly added. The resultant mixture is stirred for 30 minutes; and then 2.4 grams of para-fluorobenzonitrile is added and stirring is continued at room temperature-overnight. The reaction mixture is heated at 60° C. for 6 hours and then cooled and diluted with diethyl ether. The ether solution is washed with water and brine and is then dried over sodium sulfate and evaporated to give an oily residue. 3.3 grams of the residue is refluxed overnight with 5.0 ml. of 2N sodium hydroxide in 40 ml. of ethanol. The mixture is then concentrated to dryness, redissolved in water and washed with diethyl ether. The aqueous phase is acidired with 2N hydrochloric acid and then extracted with methylene chloride. The organic fractions are combined, dried over sodium sulfate and then evaporated to give an oil, which is Chromatographed on silica gel with methylene chloride as the eluant to yield the title compound as a crystalline product (m.p. 132°-133° C.) The sodium salt is prepared by dissolving 194 mg. of the above acid in 1.0 ml. of 1.0N sodium hydroxide. The resultant solution is evaporated to dryness, triturated with ethanol and filtered to give the sodium salt. When steps B and C of example 2 are carried out using an equivalent amount of 4-(1,1-dimethylethoxy) benzoic acid in place of 4-(2,2-dimethyl-1-oxopropyl)benzoic acid there is obtained 4-(1,1-dimethylethoxy) benzamide (m.p. 135°-136° C.).
References:
US5378728,1995,A
185259-36-3
43 suppliers
$38.00/1g

99985-67-8
22 suppliers
inquiry