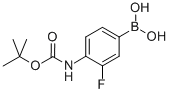
4-N-Boc-amino-3-fluorophenylboronic acid synthesis
- Product Name:4-N-Boc-amino-3-fluorophenylboronic acid
- CAS Number:218301-87-2
- Molecular formula:C11H15BFNO4
- Molecular Weight:255.05
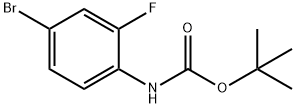
209958-42-9
65 suppliers
$30.00/1/ G

218301-87-2
78 suppliers
$69.00/500mg
Yield:218301-87-2 74%
Reaction Conditions:
Stage #1: tert-butyl N-(4-bromo-2-fluorophenyl)carbamatewith n-butyllithium in tetrahydrofuran at -85; for 1 h;
Stage #2: with Trimethyl borate in tetrahydrofuran at -90 - 20;
Stage #3: with water;acetic acidmore than 3 stages;
Steps:
A.2
Step 2The solution of the product from step 1 (25 g, 86 mmol) in THF (400 mL), was cooled to -85 0C, and then treated dropwise with 83 mL of 2.5 M n-BuLi (208 mmol). The mixture was stirred for 1 h, and quenched with trimethylborate (27 g, 260 mmol) at -90 °C. The viscous mixture was gradually warmed to rt, poured into 1 L of water, extracted with benzene (50 mL) and evaporated. The aqueous layer was neutralized with acetic acid, upon which the precipitated oil slowly started to crystallize. The precipitate was filtered off, washed with water and air-dried
References:
WO2007/147574,2007,A1 Location in patent:Page/Page column 55-56
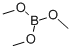
121-43-7
359 suppliers
$14.00/25mL

218301-87-2
78 suppliers
$69.00/500mg
24424-99-5
840 suppliers
$13.50/25G

218301-87-2
78 suppliers
$69.00/500mg
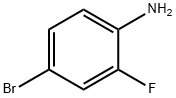
367-24-8
499 suppliers
$15.00/25g

218301-87-2
78 suppliers
$69.00/500mg