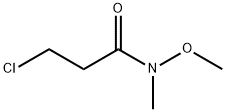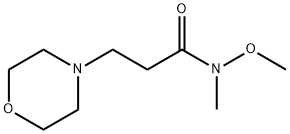
4-Morpholinepropanamide, N-methoxy-N-methyl- synthesis
- Product Name:4-Morpholinepropanamide, N-methoxy-N-methyl-
- CAS Number:405212-49-9
- Molecular formula:C9H18N2O3
- Molecular Weight:202.25
Yield:405212-49-9 46%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20;Inert atmosphere;
Steps:
4 General procedure for the preparation of compounds 4a, 5a-c and 6a
General procedure: To a solution of Weinreb amide (1.0 equiv.) in ACN, the correspondingamine (1.0 equiv.) and K2CO3 (1.5 equiv.) were added. Themixture was stirred overnight, at room temperature. After removalof the solvent under reduced pressure, the crude was extractedwith DCM (3 5 mL) and washed with water (5 mL) and brine(10 mL). In the case of compound 4a, this work-up was sufficient toobtain the pure compound. Conversely, an acid (pH 3-4)/base(pH 8-9) work-up was required for 5a-c and 6a, the combinedorganic phases were dried (anhydrous Na2SO4), filtered and,evaporated under vacuum to get the desired compounds. The crudecompound 6a was further purified through flash chromatography(silica gel) to afford pure compound 6a.
References:
Rui, Marta;Rossino, Giacomo;Coniglio, Stefania;Monteleone, Stefania;Scuteri, Arianna;Malacrida, Alessio;Rossi, Daniela;Catenacci, Laura;Sorrenti, Milena;Paolillo, Mayra;Curti, Daniela;Venturini, Letizia;Schepmann, Dirk;Wünsch, Bernhard;Liedl, Klaus R.;Cavaletti, Guido;Pace, Vittorio;Urban, Ernst;Collina, Simona [European Journal of Medicinal Chemistry,2018,vol. 158,p. 353 - 370]
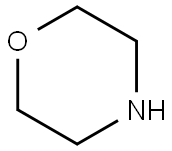
110-91-8
693 suppliers
$9.00/1g
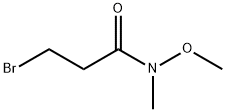
128562-58-3
16 suppliers
$45.00/10mg

405212-49-9
0 suppliers
inquiry