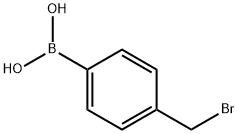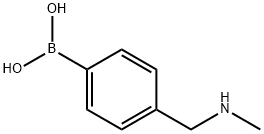
4-((MethylaMino)Methyl)phenylboronic acid synthesis
- Product Name:4-((MethylaMino)Methyl)phenylboronic acid
- CAS Number:518336-26-0
- Molecular formula:C8H12BNO2
- Molecular Weight:165
87199-17-5
430 suppliers
$8.00/5g
593-51-1
544 suppliers
$21.00/100g
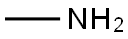
74-89-5
7 suppliers
$13.44/25ML

518336-26-0
21 suppliers
inquiry
Yield:-
Reaction Conditions:
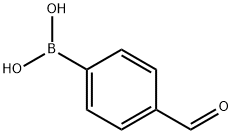
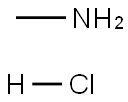
Stage #1: 4-formylphenylboronic acid,;methylamine hydrochloride;methylamine in methanol at 20; for 0.5 h;
Stage #2: with hydrogenchloride;methanol;sodium cyanoborohydride at 30; pH=4 - 6; for 2 h;
Steps:
1
A solution of 8 M methylamine in methanol (62.5 L) and methylamine hydrochloride (3.0 moles, 202.56 g) were added to methanol (3.0 L). 4-Formylphenylboronic acid (Formula- H, 1 mole) was added to the solution which was stirred at room temperature for 30 minutes. Sodium cyanoborohydride (1.3 moles, 81.7 g) was added to the reaction mixture. The pH was adjusted to 5 with methanolic HC1 and the solution was stirred for about 2 hours at about 30 °C. The pH of the reaction mixture was maintained between 4 and 6 by addition of methanolic HC1 as needed. The solvent was then removed from the reaction mixture by concentration under reduced pressure. The obtained residue was combined with water and brine to which dichloromethane was added to extract the target compound. Extractions were repeated as necessary and the combined organic fractions were concentrated and dried to yield crude (4-((methylamino)methyl)phenyl)boronic acid (Formula-I, -85% yield).
References:
WO2019/130229,2019,A1 Location in patent:Page/Page column 26

87199-17-5
430 suppliers
$8.00/5g

518336-26-0
21 suppliers
inquiry