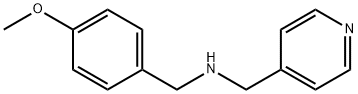
(4-METHOXY-BENZYL)-PYRIDIN-4-YLMETHYL-AMINE synthesis
- Product Name:(4-METHOXY-BENZYL)-PYRIDIN-4-YLMETHYL-AMINE
- CAS Number:418791-10-3
- Molecular formula:C14H16N2O
- Molecular Weight:228.29
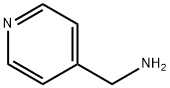
3731-53-1
287 suppliers
$6.00/5g
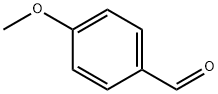
123-11-5
866 suppliers
$10.00/10g

418791-10-3
15 suppliers
$174.00/500mg
Yield:418791-10-3 38%
Reaction Conditions:
with methanol;sodium cyanoborohydride at 20; for 12 h;Molecular sieve;
Steps:
Preparation of N-(4-methoxybenzyl)-1-(pyridin-4-yl)methanamine
To a round bottom flask with a magnetic stirrer bar, 4-picolylamine (0.43 g, 4 mmol) and 4-methoxybenzaldehyde (1 .22 g, 9 mmol) were added with 10 ml methanol, NaCNBH3 (0.57 g, 9 mmol) and a few granules of 4A molecular sieve. The solution was stirred for 2-12 hours at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC). After filtration the solution was concentrated in vacuo and to the resulting liquid was added 50 ml dichloromethane and extracted with 3x50 ml 0.05 M HCI. The combined aqueous layer was made basic with NaOH until the pH 10-1 1 and the solution was extracted with 2x150 ml dichloromethane. The organic layers were combined and dried over anhydrous MgS04, filtered and evaporated to give /V-(4-methoxybenzyl)-1 -(pyridin-4- yl)methanamine as a brown oil. Yield: 0.36 g (38 %) , 1H NMR (300 MHz, CDCI3): δ 8.56 (2H, d), 7.28 (4H, m), 6.90 (2H, m), 3.82 (5H, s), 3.76 (2H, s),13C NMR (75 MHz, CDCI3): δ 158.9, 149.9, 149.6, 132, 129.4, 128.7, 123.1 , 1 14. 65. 55.4. 52.7, 51.8.
References:
WO2013/171332,2013,A2 Location in patent:Page/Page column 102; 103