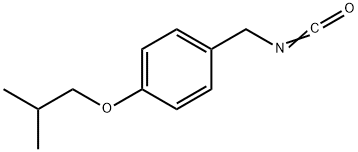
4-isobutyloxybenzyl isocyanate synthesis
- Product Name:4-isobutyloxybenzyl isocyanate
- CAS Number:639863-75-5
- Molecular formula:C12H15NO2
- Molecular Weight:205.25
![2-[4-(2-methylpropoxy)phenyl]acetic acid](/CAS/GIF/13362-94-2.gif)
13362-94-2
14 suppliers
$45.00/50mg

639863-75-5
31 suppliers
inquiry
Yield:639863-75-5 94%
Reaction Conditions:
Stage #1: 2-[4-(2-methylpropoxy)phenyl]acetic acidwith N,N,N',N'-tetramethyl-1,8-diaminonaphthalene in tetrahydrofuran at 20; for 0.416667 h;
Stage #2: with diphenyl phosphoryl azide in tetrahydrofuran at -20 - 20; for 26 h;Heating / reflux;
Steps:
1.4
Step 4; 1-Isobutoxy-4-(isocyanatomethyl)benzene: To a solution of 2-(4-Isobutoxyphenyl)acetic acid (1 g, 4.8 mmol) in anhydrous tetrahydrofuran (3 mL) was added N,N,N',N'-tetramethylnaphthalene-1,8-diamine (1.03 g, 4.8 mmol) at ambient temperature under nitrogen atmosphere. After stirring for 25 minutes, diphenylphosphorylazide (1.32 g, 4.8 mmol) was added dropwise and then the mixture was heated to reflux for 6 hours. The reaction mixture was cooled to ambient temperature and then stored at -20° C. for 20 hours to precipitate out ammonium phosphonate salt. A mixture of ether and ethyl acetate (v/v=1/1, 5 mL) was added to the cold reaction mixture. The precipitate formed was filtered off and the cake was washed with ether/ethyl acetate (1/1, v/v, 4 mL). The filtrate was concentrated in vacuo to dryness to afford the title compound (800 mg, 94% yield) which was used directly in next step without further purification.
References:
US2008/280886,2008,A1 Location in patent:Page/Page column 21-22
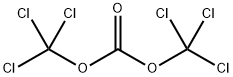
32315-10-9
425 suppliers
$10.00/1g
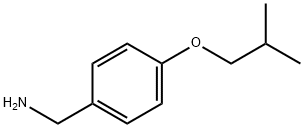
4734-09-2
82 suppliers
$45.00/1g

639863-75-5
31 suppliers
inquiry
![2-[4-(2-Methylpropoxy)phenyl]acetohydrazide](/CAS/GIF/61904-59-4.gif)
61904-59-4
8 suppliers
inquiry

639863-75-5
31 suppliers
inquiry
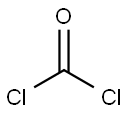
75-44-5
0 suppliers
inquiry

639863-75-5
31 suppliers
inquiry