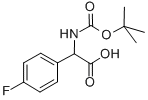
4-FLUOROPHENYLGLYCINE-N-BOC PROTECTED synthesis
- Product Name:4-FLUOROPHENYLGLYCINE-N-BOC PROTECTED
- CAS Number:142121-93-5
- Molecular formula:C13H16FNO4
- Molecular Weight:269.27
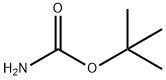
24424-99-5
840 suppliers
$13.50/25G
7292-73-1
199 suppliers
$10.00/1g

142121-93-5
16 suppliers
inquiry
Yield:142121-93-5 99%
Reaction Conditions:
with sodium hydroxide in water;isopropyl alcohol at 20; for 1 h;Cooling with ice;
Steps:
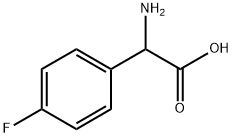
13.1 Step 1. [(tert-Butoxycarbonyl)amino](4-fluorophenyl)acetic acid
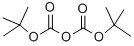
Step 1. [(tert-Butoxycarbonyl)amino](4-fluorophenyl)acetic acid To amino(4-fluorophenyl)acetic acid (from Acros, 3.0 g, 18 mmol) in 1.0 M sodium hydroxide in water (25.0 mL, 25.0 mmol), cooled in wet ice bath was added a solution of di-tert- butyldi carbonate (4.6 g, 21 mmol) in isopropyl alcohol (15.0 mL). The ice bath was removed and the resulting suspension was stirred at rt for 1 h. The volatile solvent was removed under reduced pressure and remaining solution was adjusted to pH = 3 with 4M HC1, and then extracted with Ethyl acetate. The organic fraction was washed with brine, dried over sodium sulfate, filtered and concentrated to give 4.75 g (99%) of the desired product, which was used in the next step without further purification.
References:
WO2017/27717,2017,A1 Location in patent:Page/Page column 99
124-38-9
129 suppliers
$214.00/14L
![Carbamic acid, N-[(4-fluorophenyl)(tributylstannyl)methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1358791-65-7.gif)
1358791-65-7
0 suppliers
inquiry

142121-93-5
16 suppliers
inquiry
153903-23-2
5 suppliers
inquiry