4-Ethylresorcinol synthesis
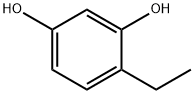
- Product Name:4-Ethylresorcinol
- CAS Number:2896-60-8
- Molecular formula:C8H10O2
- Molecular Weight:138.16
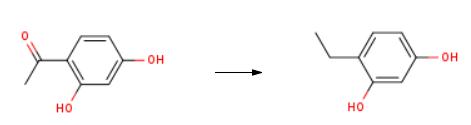
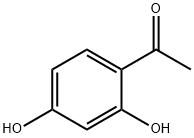
In a three necked round bottom flask (equipped with a condensor, additional funnel and mechanical stirrer) was added 15.2 g of a combination of Raney Nickel and Ni supported on silica. (50: 50). 100 ml of a mixture of 50: 50 ethanol: water was added and the reaction was heated at reflux conditions. 15.2 g of 2,4-dihydroxy acetophenone in 100 ml of water: ethanol and 10 ml of acetic acid was placed in the additional funnel and slowly added to the mixture (dropwise). The reaction was filtered through a milipore filter to give a pale yellow solution. Concentration of this solution in vacuo gave a solid. This solid was crystalized from 1,2-dichloroethane to give 4-ethyl resorcinol in 80% isolated yield.
89-84-9
551 suppliers
$6.00/10g

2896-60-8
186 suppliers
$45.00/250mg
Yield:2896-60-8 80%
Reaction Conditions:
with hydrogen;acetic acid;Ni-doped silica in ethanol;water
Steps:
5
In a three necked round bottom flask (equipped with a condensor, additional funnel and mechanical stirrer) was added 15.2 g of a combination of Raney Nickel and Ni supported on silica. (50: 50). 100 ml of a mixture of 50: 50 ethanol: water was added and the reaction was heated at reflux conditions. 15.2 g of 2,4-dihydroxy acetophenone in 100 ml of water: ethanol and 10 ml of acetic acid was placed in the additional funnel and slowly added to the mixture (dropwise). The reaction was filtered through a milipore filter to give a pale yellow solution. Concentration of this solution in vacuo gave a solid. This solid was crystalized from 1,2-dichloroethane to give 4-ethyl resorcinol in 80% isolated yield. The unreacted 2,4-dihydroxy acetophenone was recovered (-17%) form the reaction mixture and was recycled. The structure of 4-ethyl resorcinol and 2,4- dihydroxy acetophenone were characterized by NMR, Gas Chromatography, IR and Mass spectroscopy Examples 6 to 16: The conditions under which examples 6 to 16 were carried out are summarized in Table-1. The procedure under which these experiments were carried out are also given in the table-1 and these procedures are given below as procedure 1 and procedure 2.
References:
UNILEVER PLC;UNILEVER NV;HINDUSTAN LEVER LIMITED WO2005/5355, 2005, A1 Location in patent:Page/Page column 13-14

100-41-4
351 suppliers
$5.00/5 g

2896-60-8
186 suppliers
$45.00/250mg
106209-27-2
3 suppliers
inquiry

2896-60-8
186 suppliers
$45.00/250mg
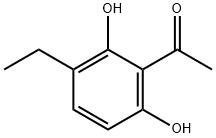
54337-59-6
6 suppliers
inquiry

2896-60-8
186 suppliers
$45.00/250mg

89-84-9
551 suppliers
$6.00/10g

2896-60-8
186 suppliers
$45.00/250mg
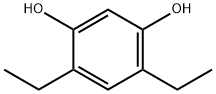
52959-32-7
2 suppliers
inquiry
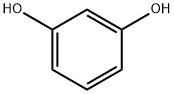
108-46-3
774 suppliers
$10.00/10g