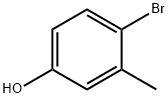
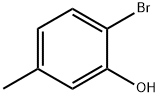
4-Bromo-3-methylphenol synthesis
- Product Name:4-Bromo-3-methylphenol
- CAS Number:14472-14-1
- Molecular formula:C7H7BrO
- Molecular Weight:187.03
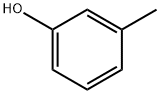
108-39-4
510 suppliers
$13.00/25g

14472-14-1
266 suppliers
$10.00/5g
Yield:14472-14-1 96%
Reaction Conditions:
with o-xylylene bis(triethylammonium tribromide) in acetonitrile at 20; for 0.0833333 h;regioselective reaction;
Steps:
3.3. General Procedure for Bromination of AromaticCompounds
General procedure: To a magnetic solution of aromatic compound (1 mmol)in acetonitrile (5 mL), OXBTEATB (0.233 g, 0.5 mmol) wasadded and stirred at room temperature for the appropriatetime (Table 1). The reaction was monitored by TLC (eluent:n-hexane/ethyl acetate: 5/1). The reaction mixture was transferredinto a separatory funnel after filtration of OXBTEABand was extracted with water (15 mL) and dichloromethane(20 mL). The organic layer was dried over anhydrousNa2SO4, and the solvent was concentrated in a rotary evaporator.The crude product was purified by passing it over acolumn of silica gel using a mixture of n-hexane and ethylacetate as the eluent. In order to regenerate the reagent, whitesolid was treated with liquid bromine. All the product structureswere confirmed by comparison of melting point or 1HNMR spectra with ones reported in the literature [29a-29e].
References:
Hemati, Roya;Shahvelayati, Ashraf S.;Yadollahzadeh, Khadijeh [Letters in Organic Chemistry,2018,vol. 15,# 8,p. 682 - 687]

108-39-4
510 suppliers
$13.00/25g

14472-14-1
266 suppliers
$10.00/5g
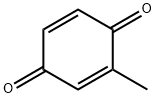
553-97-9
198 suppliers
$5.00/5g
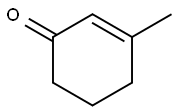
1193-18-6
174 suppliers
$6.00/1g

14472-14-1
266 suppliers
$10.00/5g

1193-18-6
174 suppliers
$6.00/1g

14472-14-1
266 suppliers
$10.00/5g

108-39-4
510 suppliers
$13.00/25g

108-39-4
510 suppliers
$13.00/25g

14472-14-1
266 suppliers
$10.00/5g
14847-51-9
124 suppliers
$9.00/100mg