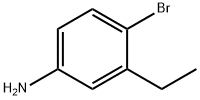
4-Bromo-3-ethylaniline synthesis
- Product Name:4-Bromo-3-ethylaniline
- CAS Number:52121-42-3
- Molecular formula:C8H10BrN
- Molecular Weight:200.08
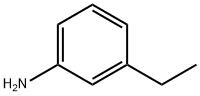
587-02-0
171 suppliers
$10.00/1g

52121-42-3
23 suppliers
$24.00/250mg
Yield:52121-42-3 89.9%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0 - 25; for 1 h;
Steps:
1 Preparation of compound 22 (see, FIG. 7):
To a solution of compound 21 (1.00 gram, 8.25 mmol, 1.03 mL, 1.00 mol equivalent) in DMF (10 mL) was added NBS (1.47 gram, 8.25 mmol, 1.00 mol equivalent) at 0 °C. The reaction mixture was stirred at 25 °C for 1 hour. TLC (petroleum ether/ethyl acetate = 10/1) showed that compound 21 (Rf = 0.32) was consumed and a new spot (Rf = 0.27) was formed. The reaction mixture was diluted with ethyl acetate (50 mL), washed with brine (50 mL x 3). The organic layer was dried over Na2SO4, filtered and concentrated under vacuum to give a residue, which was purified by flash silica gel chromatography (ISCO; 2.3 g SepaFlash Silica Flash Column, Eluent of 0-10% ethyl acetate/petroleum ether, TLC: petroleum ether/ethyl acetate = 10/1, Rf = 0.27) to give compound 22 (1.50 gram, 7.42 mmol, 89.9 % yield, 99.0 % purity) as a brown liquid, as confirmed by LCMS (not shown).
References:
WO2022/34587,2022,A1 Location in patent:Page/Page column 94; 96
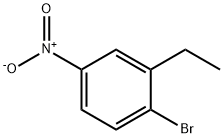
52121-40-1
2 suppliers
inquiry

52121-42-3
23 suppliers
$24.00/250mg