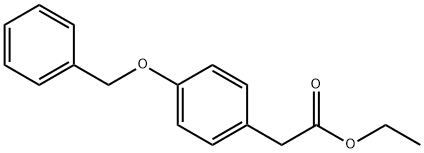
4-BENZYLOXYPHENYLACETIC ACID ETHYL ESTER synthesis
- Product Name:4-BENZYLOXYPHENYLACETIC ACID ETHYL ESTER
- CAS Number:56441-69-1
- Molecular formula:C17H18O3
- Molecular Weight:270.32
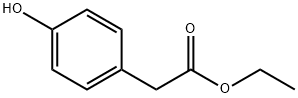
17138-28-2
209 suppliers
$14.00/1g
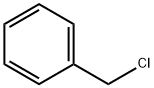
100-39-0
433 suppliers
$10.00/10g

56441-69-1
49 suppliers
$90.00/1g
Yield:56441-69-1 89%
Reaction Conditions:
with sodium hydride;tetra-(n-butyl)ammonium iodide in tetrahydrofuran at 0 - 20; for 18 h;
Steps:
121 Reference Example 121; Ethyl 2-(4-benzyloxyphenyl)acetate (I-121)
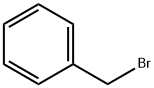
To tetrahydrofuran (75 ml) suspension of 639 mg (16.0 mmol) of sodium hydride were added tetrahydrofuran (30 ml) solution of 2.00 g (11.1 mmol) of ethyl (4-hydroxyphenyl) acetate and tetrahydrofuran (30 ml) solution of 491 mg (1.33 mmol) of tetrabutylammonium iodide and 2.37 ml (20.0 mmol) of benzyl bromide under nitrogen atmosphere at 0°C, and stirred at room temperature for 18 hours. Water and aqueous saturated ammonium chloride solution were added thereto, and extracted with ethyl acetate. The combined ethyl acetate layers were dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The resulting residue was applied to a silica gel column chromatography. From the eluate with n-hexane/ethyl acetate (5/1, v/v), 2.67 g (89 %) of the entitled compound was obtained as a colorless oil. MS(ESI)m/z:271(M+1)+.1H-NMR(CDCl3)δ: 1.20-1.29(3H, m), 3.55(2H, s), 4.14(2H, q, J=7.1Hz), 5.05(2H, s), 6.89-6.98(2H, m), 7.15-7.27(3H, m), 7.30-7.46 (4H, m). IR(ATR): 1730, 1510, 1454, 1298, 1236, 1221, 1176, 1149, 1026 cm-1.
References:
EP1479681,2004,A1 Location in patent:Page 83

17138-28-2
209 suppliers
$14.00/1g
100-44-7
644 suppliers
$13.50/250G

56441-69-1
49 suppliers
$90.00/1g
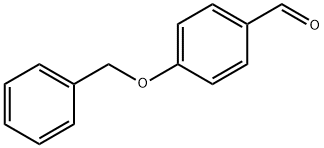
4397-53-9
327 suppliers
$8.00/10g

56441-69-1
49 suppliers
$90.00/1g

![Benzene, 1-[2-(methylsulfinyl)-2-(methylthio)ethenyl]-4-(phenylmethoxy)-](/CAS/20210305/GIF/71749-20-7.gif)