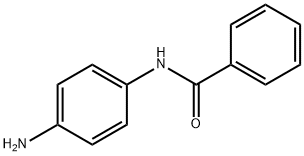
4'-Aminobenzanilide synthesis
- Product Name:4'-Aminobenzanilide
- CAS Number:17625-83-1
- Molecular formula:C13H12N2O
- Molecular Weight:212.25
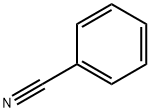
100-47-0
372 suppliers
$14.14/25gm:
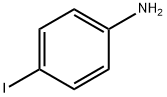
540-37-4
448 suppliers
$6.00/5g

17625-83-1
143 suppliers
$13.00/500mg
Yield: 85%
Reaction Conditions:
with copper(l) iodide;Acetaldehyde oxime;potassium carbonate;N,N`-dimethylethylenediamine in toluene at 120; for 15 h;Inert atmosphere;Schlenk technique;
Steps:
4.2 General procedure for the synthesis of N-arylamides
General procedure: To a suspension of CuI (9.5 mg, 0.05 mmol), K2CO3 (138.2 mg, 1.0 mmol) in toluene (2.0 mL) were added aryl monohalides 1 (0.5 mmol), nitrile 2 (1.0 mmol), DMEDA (8.0 μL, 0.075 mmol), acetaldoxime (92.0 μL, 1.5 mmol) under argon. The reaction mixture was stirred for 15h at 120°C under argon. The solution was cooled to room temperature and the solvent was removed under vacuum. The crude product was purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate=5:1) to afford the N-arylamides 3.
References:
Zhang, Dong-Xue;Xiang, Shi-Kai;Hu, Hao;Tan, Wen;Feng, Chun;Wang, Bi-Qin;Zhao, Ke-Qing;Hu, Ping;Yang, Hua [Tetrahedron,2013,vol. 69,# 47,p. 10022 - 10029]
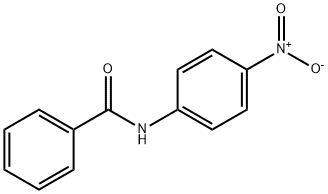
3393-96-2
84 suppliers
$30.00/20mg

17625-83-1
143 suppliers
$13.00/500mg
![Carbamic acid, N-[4-(benzoylamino)phenyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/914489-91-1.gif)
914489-91-1
0 suppliers
inquiry

17625-83-1
143 suppliers
$13.00/500mg
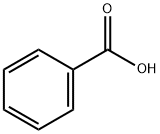
65-85-0
1383 suppliers
$10.00/25 g
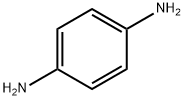
106-50-3
619 suppliers
$19.00/25g

17625-83-1
143 suppliers
$13.00/500mg
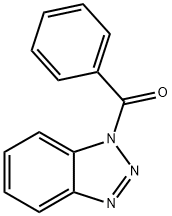
4231-62-3
32 suppliers
$83.40/1g

106-50-3
619 suppliers
$19.00/25g

17625-83-1
143 suppliers
$13.00/500mg