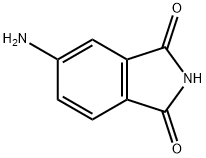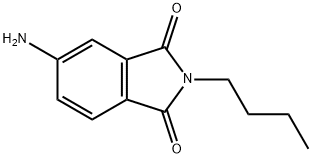
4-AMINO-N-BUTYL PHTHALIMIDINE synthesis
- Product Name:4-AMINO-N-BUTYL PHTHALIMIDINE
- CAS Number:68930-97-2
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25
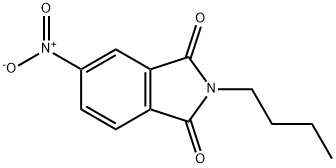
54395-37-8
31 suppliers
inquiry

68930-97-2
48 suppliers
inquiry
Yield:68930-97-2 750 mg (59%)
Reaction Conditions:
with sodium carbonate in methanol;water;
Steps:
4-[3-(2-Butyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-1-(4-tert-butylphenyl)ureidomethyl]-N-(2H-tetrazol-5-yl)benzamide
A solution of N-butyl-4-nitrophthalimide (1.45 g, 5.8 mmol) in methanol (30 mL) was added drop wise to a well stirred solution of sodium dithionite (6.50 g, 37.1 mmol) and sodium carbonate (3.22 g, 30.5 mmol) in water (40 mL), while the temperature was maintained at 70° C. After addition, heating at 70° C. was continued for a further 30 minutes, then the reaction mixture was allowed to cool to room temperature. The reaction volume was reduced to one third by rotary evaporation, and the residual water solution was extracted with diethyl ether (2*50 mL). The combined organic phases were dried with anhydrous Na2SO4, and then taken to dryness. The residual oil was recrystallized from ethanol/water to give 750 mg (59%) of N-butyl-4-aminophthalimide. 1H NMR (DMSO-d6): δ0.89 (t, 3H); 1.26 (m, 2H); 1.53 (m, 2H); 3.48 (t, 2H); 6.44 (bs, 2H); 6.78 (dd, 1H); 6.92 (d,1H); 7.46 (d, 1H).
References:
US6503949,2003,B1
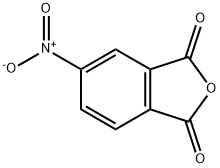
5466-84-2
238 suppliers
$11.00/1g

68930-97-2
48 suppliers
inquiry