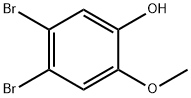
4,5-dibromo-2-methoxyphenol synthesis
- Product Name:4,5-dibromo-2-methoxyphenol
- CAS Number:38926-86-2
- Molecular formula:C7H6Br2O2
- Molecular Weight:281.93
2563-26-0
70 suppliers
$90.00/500mg

74-88-4
349 suppliers
$15.00/10g

38926-86-2
6 suppliers
$12.00/1mg
Yield:38926-86-2 42%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 22 h;Inert atmosphere;
Steps:
4,5-Dibromo-2-methoxyphenol (4). To a solution of dibromocatechol36) (8.10 g, 30.2 mmol) in DMF (91 mL) were added K2CO3 (4.13 g, 30.2 mmol) and MeI (1.9 mL, 30.6 mmol) at room temperatureu nder nitrogen. After being stirred at room temperature for 22 h, the reaction was quenched with H2O. The aqueous layer was extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated. The residue was purified by silica gel column chromatography (hexane/EtOAc 12:1 to 6:1 to1:1) to give 4 (3.57 g, 42%) as a white powder. Mp 91-92 °C (lit.16) 92-93 °C); IR (KBr) max (cm1): 3388, 1492, 1433, 1264, 1203, 1027,865, 797; 1H-NMR (CDCl3, 300 MHz) (ppm): 7.18 (1H, s, Ar), 7.06(1H, s, Ar), 5.57 (1H, s, -OH), 3.88 (3H, s, -OMe); 13C-NMR (CDCl3,100 MHz) (ppm): 146.4, 145.6, 119.1, 115.5, 115.4, 113.8, 56.3;LR-MS (ESI, negative): calcd. for C7H5Br2O2 (M-H), 279; found,279. The NMR data for 4 are identical to the reported data.16)
References:
Nakazaki, Atsuo;Huang, Wen-Yu;Koga, Kazushi;Yingyongnarongkul, Boon-Ek;Boonsombat, Jutatip;Sawayama, Yusuke;Tsujimoto, Takashi;Nishikawa, Toshio [Bioscience, Biotechnology and Biochemistry,2013,vol. 77,# 7,p. 1529 - 1532]
90-05-1
752 suppliers
$5.00/1g

38926-86-2
6 suppliers
$12.00/1mg
35488-17-6
0 suppliers
inquiry

38926-86-2
6 suppliers
$12.00/1mg
37942-01-1
248 suppliers
$6.00/5g

38926-86-2
6 suppliers
$12.00/1mg

613-70-7
117 suppliers
$30.00/1g

38926-86-2
6 suppliers
$12.00/1mg