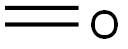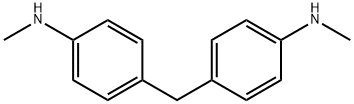
4,4'-METHYLENEBIS(N-METHYLANILINE) synthesis
- Product Name:4,4'-METHYLENEBIS(N-METHYLANILINE)
- CAS Number:1807-55-2
- Molecular formula:C15H18N2
- Molecular Weight:226.32
52721-83-2
5 suppliers
$28.60/250mg

1807-55-2
8 suppliers
inquiry
Yield:1807-55-2 543 mg
Reaction Conditions:
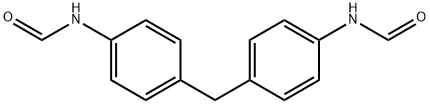
Stage #1: N,N'-(methylenebis(4,1-phenylene))diformamidewith lithium aluminium tetrahydride in tetrahydrofuran; for 24 h;Inert atmosphere;Reflux;
Stage #2: with Glauber's salt in tetrahydrofuran; for 1 h;Inert atmosphere;Reflux;
Steps:
Synthesis of N,N'-Dimethyl-N,N'-(methylenedi-4,1-phenylene)bis[2-(1-pyrrolidinyl)acetamide] (2g)
To a solution of 2a (793 mg, 4.0 mmol) in THF (4 mL) was added ethyl formate (937 mg, 12 mmol) at 25 °C, and the mixture was stirred at 55 °C for 48 h. Removal of the solvent under reduced pressure gave crude N,N'-(methylenedi-4,1-phenylene)bisformamide (997 mg) as a colorless solid. To a suspension of LiAlH4 (455 mg, 12 mmol) in THF (40 mL) was added N,N'-(methylenedi-4,1-phenylene)bisformamide (997 mg) at 0 °C, and the mixture was stirred under reflux for 24 h. To the reaction mixture was added Na2SO4?10H2O (6.44 g, 20 mmol) at 0 °C, and the mixture was stirred under reflux for 1 h. After the insoluble parts were filtered, the filtrate was concentrated under reduced pressure. The residue was purified by MPLC on silica gel using hexane/AcOEt (1 : 1) as eluent to give N,N'-dimethyl-4,4'-diaminodiphenylmethane (543 mg, 2.40 mmol, 60%) as pale yellow oil. To a solution of N,N'-dimethyl-4,4'-diaminodiphenylmethane (113 mg, 0.50 mmol), pyridine (95 mg, 1.20 mmol), and 4-(dimethylamino)pyridine (1 mg) in CH2Cl2 (10 mL) was added bromoacetyl bromide (222 mg, 1.10 mmol) at 0 °C, and the mixture was stirred at 25 °C for 3 h. The reaction mixture was poured into water (10 mL), and the organic layer was dried over Na2SO4, and filtered. Removal of the solvent under reduced pressure gave crude N,N'-dimethyl-N,N'-(methylenedi-4,1-phenylene)bis(2-bromoacetamide) (200 mg) as pale yellow oil. To a suspension of N,N'-dimethyl-N,N'-(methylenedi-4,1-phenylene)bis(2-bromoacetamide) (200 mg) and K2CO3 (276 mg, 2.0 mmol) in THF (5.0 mL) was added pyrrolidine (107 mg, 1.50 mmol) at 25 °C, and the mixture was stirred at 60 °C for 12 h. The reaction mixture was poured into water (10 mL) and extracted with CHCl3 (10 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by MPLC on silica gel using CHCl3/MeOH (19 : 1) as eluent to give 2g (178 mg, 0.40 mmol, 79%) as a pale yellow solid.
References:
Kimura, Tsutomu;Hosokawa-Muto, Junji;Kamatari, Yuji O.;Kuwata, Kazuo [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 5,p. 1502 - 1507] Location in patent:supporting information; experimental part
920-66-1
645 suppliers
$6.00/10g
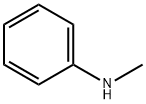
100-61-8
405 suppliers
$8.00/1g

1807-55-2
8 suppliers
inquiry
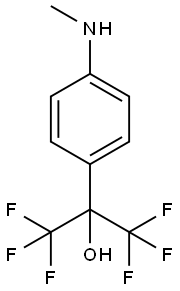
1481-11-4
8 suppliers
inquiry
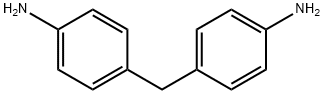
101-77-9
363 suppliers
$15.00/25g
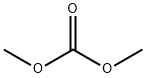
616-38-6
706 suppliers
$5.00/5G

1807-55-2
8 suppliers
inquiry
![N,N-Dimethyl-4-[[4-(methylamino)phenyl]methyl]benzenamine](/CAS/GIF/53477-27-3.gif)
53477-27-3
0 suppliers
inquiry
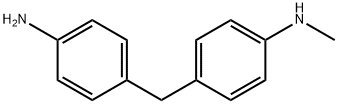
26628-67-1
12 suppliers
$650.75/1G
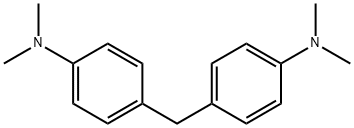
101-61-1
192 suppliers
$7.00/25g