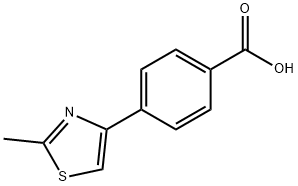
4-(2-METHYL-1,3-THIAZOL-4-YL)BENZOIC ACID synthesis
- Product Name:4-(2-METHYL-1,3-THIAZOL-4-YL)BENZOIC ACID
- CAS Number:294620-60-3
- Molecular formula:C11H9NO2S
- Molecular Weight:219.26
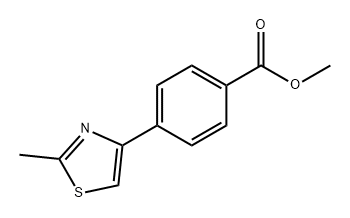
805994-92-7
2 suppliers
inquiry

294620-60-3
38 suppliers
$65.00/100mg
Yield:294620-60-3 94%
Reaction Conditions:
with lithium hydroxide;water in tetrahydrofuran;ethanol at 20; for 16 h;
Steps:
B.16 Preparation 16; 4-(2-Methyl-thiazol-4-yl)-benzoic acid
A mixture of methyl-4-acetylbenzoate (7.59 g, 43 mmol) and acetic acid (100 mL) w heated to 70° C. The reaction was cooled to 30° C. once the benzoate was dissolved. Bromine (2.2 mL, 43 mmol) was added dropwise over a period of 30 mins. The reaction was stirred at ambient temperature under nitrogen for 19 hrs. Precipitates formed upon cooling with an ice bath. The precipitate was filtered and washed with a cold solution of 1:1 MeOH/H2O. The crude material (5.05 g) was taken to the next reaction without further purification. A solution of 4-bromoacetyl-benzoic acid methyl ester (0.50 g, 1.9 mmol), ethanol (6.5 mL), and thioacetamide (0.29 mg, 3.9 mmol) was refluxed under nitrogen for 5 hrs. After cooling, the reaction was poured onto water (75 mL) and stirred for 10 mins. The precipitate was filtered and washed with water. The product was collected as a pale yellow powder 0.43 g (95%). A solution of 4-(2-methyl-thiazol-4-yl)-benzoic acid methyl ester (400 mg, 1.7 mmol), lithium hydroxide (108 mg, 2.6 mmol), tetrahydrofuran (2 mL), water (2 mL), and methanol (1.5 mL) was stirred at ambient temperature under nitrogen for 16 hrs. The reaction was concentrated, diluted with water (10 mL), acidified with HCl (4 mL, 1M), and extracted with ethyl acetate and methylene chloride. The organic layers were combined, dried on sodium sulfate, and concentrated to give 0.35 g (94%) of 4-(2-methyl-thiazol-4-yl)-benzoic acid, which was used in the preparation of compound 47 of Table 1.
References:
US2004/248949,2004,A1 Location in patent:Page 35-36
66047-74-3
43 suppliers
$40.00/250mg
201230-82-2
1 suppliers
inquiry

294620-60-3
38 suppliers
$65.00/100mg
3609-53-8
201 suppliers
$5.00/1g

294620-60-3
38 suppliers
$65.00/100mg
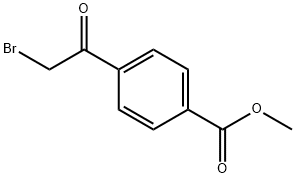
56893-25-5
79 suppliers
$50.75/250mg

294620-60-3
38 suppliers
$65.00/100mg
99-73-0
233 suppliers
$10.00/1g

294620-60-3
38 suppliers
$65.00/100mg