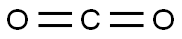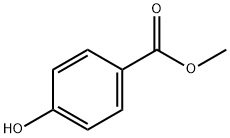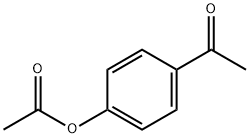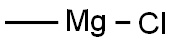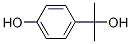
4-(2-hydroxypropan-2-yl)phenol synthesis
- Product Name:4-(2-hydroxypropan-2-yl)phenol
- CAS Number:2948-47-2
- Molecular formula:C9H12O2
- Molecular Weight:152.19
Yield:2948-47-2 98.5%
Reaction Conditions:
with ammonium chloride;methyllithium in tetrahydrofuran;ice-water;diethyl ether;hexane;ethyl acetate;acetone;
Steps:
1 Example 1
Example 1 This example provides a description of the preparation of 2-(4-hydroxyphenyl)-2-propanol, which is then used to prepare bisphenol A in some embodiments of the present invention. A stirred mixture of methyl lithium (537 ml, 0.564 mol, 1.05M) and diethyl ether (250 ml) was cooled to -78° C. under a blanket of argon gas, using a dry ice/acetone cooling system. A solution of methyl-4-hydroxybenzoate (20 g, 0.131 mol, from Aldrich Chemical Company) in diethyl ether (50 ml) and tetrahydrofuran (50 ml) was added, drop-wise, to the methyl lithium/diethyl ether mixture. The reaction was gradually warmed to room temperature over 12 hours. The reaction was then gently refluxed over a period of 2 hours. The reaction mixture was then cooled to 0° C., using an ice-water mixture, and quenched with the drop-wise addition of a saturated ammonium chloride solution (250 ml). The mixture was partitioned, and the aqueous layer was washed once with ethyl acetate (200 ml). The organic extracts were combined and washed twice with water (200 ml), and then washed with saturated brine (200 ml). The extracts were then dried over magnesium sulfate, filtered and concentrated, yielding an orange semi-solid. The solid was recrystallized twice from a mixture of diethyl ether/ethyl acetate/hexane (1:1:1 by volume). Two crops were collected and dried, producing 5.5 g (28%) (unoptomized) yield of 2-(4-hydroxyphenyl)-2-propanol. The product was 98.5% pure, as determined by high pressure liquid chromatography (HPLC). Hydrogen proton NMR and UV spectra were consistent with the desired product.
References:
US5990362,1999,A
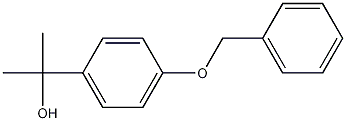
94571-13-8
7 suppliers
inquiry

2948-47-2
13 suppliers
inquiry
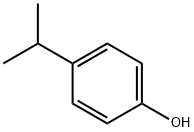
99-89-8
194 suppliers
$13.00/10g
4286-23-1
107 suppliers
$34.00/100mg

2948-47-2
13 suppliers
inquiry