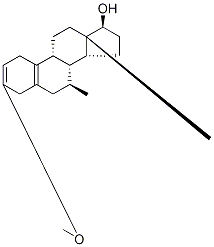
3-Methoxy-7α-Methyl-estra-2,5(10)-dien-17β-ol synthesis
- Product Name:3-Methoxy-7α-Methyl-estra-2,5(10)-dien-17β-ol
- CAS Number:15506-02-2
- Molecular formula:C20H30O2
- Molecular Weight:302.45

15506-01-1
5 suppliers
$650.00/20mg

15506-02-2
7 suppliers
$2440.00/200mg
Yield:15506-02-2 75%
Reaction Conditions:
with ammonia;calcium;isopropyl alcohol in tetrahydrofuran;tert-butyl methyl ether at -38;Birch Reduction;
Steps:
5 Example 5
Liquid ammonia (383.51 g) was added a reaction vessel containing a SLURRY of compound (7A) (150 g), in tetrahydrofuran (266. 70 g), TEST-BUTYL methyl ether (888 G) and 2-propanol (235.5 g) AT-385 C. Calcium metal (60. 12 g) was then added portion-wise and the reaction mixture was stirred AT-385 C until the blue coloration dissipated completely. The reaction is monitored by HPLC. On completion, the reaction was quenched by addition of ammonium CHLORIDE (1Y9. 51 G) AND THEN WARMED TO-10 TO 0 C. ANY AMMONIA DISTILLED was trapped in a water scrubber. Water (1. 2 kg) was added to the resulting off-white suspension and the mixture was warmed to ambient temperature. The organic layer was separated and the aqueous layer was RE-EXTRACTED with heptane. The combined organic extracts were washed with 5% W/V aqueous ammonium chloride solution, 1% W/V aqueous sodium hydrogen carbonate and water. The organic phase was concentrated under reduced pressure down to 6 volumes with respect to the weight of compound (7) input. A mixture of, heptane (153.9 g) and TERT-BUTANOL (58. 5 g) was added and distillation was continued until level of TERT-BUTYL METHYL ether was No.12. 5% w/w. The resulting suspension was chilled to 0-5 C, filtered and the product washed with heptane and dried at 25-30 C to constant weight (yield : 75%).
References:
WO2004/78774,2004,A1 Location in patent:Page 36
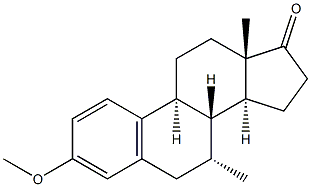
10449-00-0
0 suppliers
inquiry

15506-02-2
7 suppliers
$2440.00/200mg