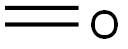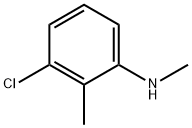
3-Chloro-N,2-dimethylaniline synthesis
- Product Name:3-Chloro-N,2-dimethylaniline
- CAS Number:41456-52-4
- Molecular formula:C8H10ClN
- Molecular Weight:155.62
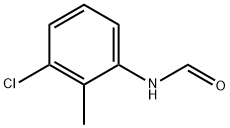
71862-02-7
21 suppliers
$28.60/250mg

41456-52-4
28 suppliers
$105.00/1g
Yield:41456-52-4 79%
Reaction Conditions:
Stage #1: N-(3-chloro-2-methylphenyl)formamidewith borane-THF in tetrahydrofuran at -40 - 20;
Stage #2: with hydrogenchloride;water in tetrahydrofuran at 20;
Stage #3: with sodium hydroxide in water;acetonitrile;
Steps:
11.b
(b) (3-Chloro-2-methylphenyl)methanamine; Borane-tetrahydrofuran (1 M in THF, 61 mL, 61 mmol) was added dropwise to λ/-(3-chloro-2-methylphenyl)formamide (3.43 g, 20.2 mmol; see step (a) above) in THF (25 mL) at -40 0C. The mixture was allowed to warm to rt and HCI (aq, 0.1 M) was added. The mixture was extracted with MeOfBu and the combined extracts were washed with water and brine, dried over Na2SO4 and concentrated. The residue was dissolved in Et2O (30 mL) and HCI (4 M in dioxane, 5.5 mL) was added. The precipitate was collected and dissolved in acetonitrile (5 mL). NaOH (aq, 2 M, 15 mL) was added and the mixture was extracted with MeOfBu. The combined extracts were washed with water, brine and dried over Na2SO4. Concentration and vacuum distillation afforded the sub-title compound (2.5 g, 79% yield) as a colourless oil.
References:
WO2008/129288,2008,A2 Location in patent:Page/Page column 57
70289-22-4
0 suppliers
inquiry

41456-52-4
28 suppliers
$105.00/1g
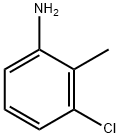
87-60-5
446 suppliers
$8.00/25g

41456-52-4
28 suppliers
$105.00/1g
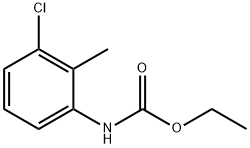
72290-16-5
0 suppliers
inquiry

41456-52-4
28 suppliers
$105.00/1g