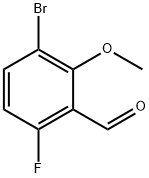
3-bromo-6-fluoro-2-methoxybenzaldehyde synthesis
- Product Name:3-bromo-6-fluoro-2-methoxybenzaldehyde
- CAS Number:473416-74-9
- Molecular formula:C8H6BrFO2
- Molecular Weight:233.03
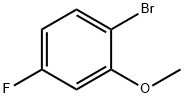
450-88-4
223 suppliers
$10.00/1g

473416-74-9
40 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
Stage #1: with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 1.16667 h;
Stage #2: 1-bromo-4-fluoro-2-methoxybenzenewith N-Formylpiperidine in water;acetic acid at 20; for 1.91667 h;
Steps:
II-11-a PRODUCTION EXAMPLE II-11-a3-Bromo-6-fluoro-2-methoxy-benzaldehyde
In an atmosphere of nitrogen gas, 18.7 ml of a 2.66 M solution of n-butyllithium in hexane was added to a solution of 5 g of N,N-diisopropylamine in 89 ml tetrahydrofuran at -78C. After stirring at the same temperature for 1 hour and 10 minutes, 9.27 g of 1-bromo-4-fluoro-2-methoxy-benzene obtained in Production Example II-1-a was added dropwise. After stirring at the same temperature for 1.5 hours, 5.52 ml of N-formylpiperidine was added dropwise. After stirring at the same temperature for 25 minutes, 9 ml of acetic acid was added at the same temperature, water was added at room temperature, and the mixture was extracted with diethyl ether for three times. The extract was sequentially washed with 0.2 N hydrochloric acid, water and brine, dried over anhydrous magnesium sulfate and the solvent was evaporated. The crude product was purified and separated by silica gel column chromatography, to give 5.65 g of the title compound as pale yellow crystals.1H-NMR ( 400 MHz, CDCl3 ) d 3.97 ( 3H, s ), 6. 90 (1H, dd, J = 9.0, 9.6 Hz ), 7.76 ( 1H, dd, J = 5.6, 9.0 Hz ), 10.35 ( 1H, s )
References:
EP1380576,2004,A1 Location in patent:Page 102
147460-41-1
269 suppliers
$5.00/1g

473416-74-9
40 suppliers
$45.00/100mg