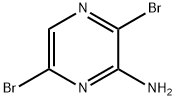
3,6-dibromopyrazin-2-amine synthesis
- Product Name:3,6-dibromopyrazin-2-amine
- CAS Number:957230-70-5
- Molecular formula:C4H3Br2N3
- Molecular Weight:252.89


957230-68-1
70 suppliers
$35.00/1g

957230-70-5
113 suppliers
$20.00/250mg
Yield:-
Reaction Conditions:
Stage #1:3,6-dibromo-pyrazine-2-carboxylic acid with diphenyl phosphoryl azide;triethylamine in tert-butyl alcohol for 18 h;Reflux;
Stage #2: with trifluoroacetic acid in dichloromethane for 0.5 h;
Stage #3: with sodium hydroxide in dichloromethane;water
Steps:
1.2
Step 2: Synthesis of Compound (C) as Described in the General Reaction Scheme; 3,6-Dibromo-pyrazin-2-ylamine Diphenylphosphorylazide (2.59 1 mL, 12 mmol) and triethylamine (1.67 mL, 12 mmol) are added to a solution of 3,6-dibromo-pyrazin-2-carboxylic acid (3.52 g, 12 mmol) in t-butanol (90 mL). The reaction is heated at reflux for 18 hours. The reaction is quenched with water, then concentrated in vacuo and taken up in DCM. The organic solution is washed with water and 1N NaOH, dried over MgSO4 and concentrated in vacuo. The resultant solid is filtered through a pad of silica using EtOAc, then concentrated and TFA:DCM (4:1, 12 mL) is added to the solid and stirred for 30 min. The solution is concentrated in vacuo then neutralised with 1N NaOH and extracted with DCM. The organic layer is dried over anhydrous MgSO4 and concentrated in vacuo to give the product. 1H NMR (250 MHz, DMSO-d6) δ (ppm) 7.25 (br s, 2H), 7.68 (s, 1H); m/z (APCI) 254 (M+H)+; m.p 135-139° C.
References:
Andrews, Martin James Inglis;Chambers, Mark Stuart;Van De Po?l, Hervé;Bar, Grégory Louis Joseph US2009/286798, 2009, A1 Location in patent:Page/Page column 26

1082843-68-2
0 suppliers
inquiry

957230-70-5
113 suppliers
$20.00/250mg
1301613-77-3
21 suppliers
$82.59/250mg

957230-70-5
113 suppliers
$20.00/250mg