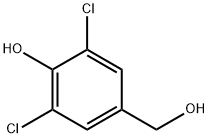
3,5-DICHLORO-4-HYDROXYBENZYL ALCOHOL synthesis
- Product Name:3,5-DICHLORO-4-HYDROXYBENZYL ALCOHOL
- CAS Number:22002-17-1
- Molecular formula:C7H6Cl2O2
- Molecular Weight:193.03
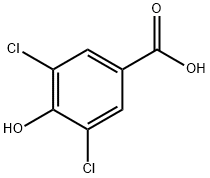
3336-41-2
180 suppliers
$9.00/1g

22002-17-1
24 suppliers
$432.00/1g
Yield:22002-17-1 96%
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 13.25 h;
Steps:
BH3 (1M in THF, 250 mL, 250 mmol) was added dropwise to a cooled sol. of 3,5-dichloro-4-hydroxybenzoic acid (20 g, 96.6 mmol) in THF (200 mL) at 0 °C. The resulting mixture was stirred at 0 °C for 15 min., and then at rt for 13 h. The milky mixture was cooled to 0 °C, and MeOH (150 mL), then water (100 mL), were added dropwise. The mixture was further stirred at 0 °C for 15 min, and then at rt for 5 h. The mixture was then partially concentrated under reduced pressure. EtOAc (200 mL) and water (50 mL) were added to the residue, and the phases were shaken and separated. Theaq. phase was further extracted with EtOAc. The combined org. extracts were washed with brine, dried over MgSC^, filtered, and concentrated under reduced pressure. Purification by FC (CH2Cl2/CH3OH, 100:1) led to the title compound as a slightly beige solid (17.86 g, 96%). LC-MS: tR = 0.69 min.
References:
WO2006/21402,2006,A1 Location in patent:Page/Page column 37-38
3337-59-5
113 suppliers
$6.00/250mg

22002-17-1
24 suppliers
$432.00/1g
17302-82-8
120 suppliers
$10.00/1g

22002-17-1
24 suppliers
$432.00/1g
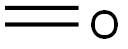
50-00-0
885 suppliers
$10.00/25g
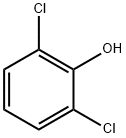
87-65-0
413 suppliers
$10.00/10g

22002-17-1
24 suppliers
$432.00/1g
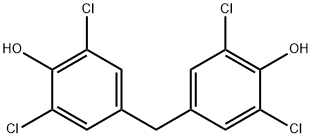
3933-88-8
4 suppliers
inquiry
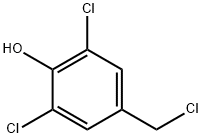
45952-61-2
0 suppliers
inquiry

22002-17-1
24 suppliers
$432.00/1g