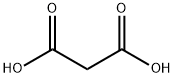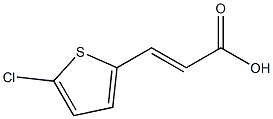
3-(5-chlorothiophen-2-yl)acrylic acid synthesis
- Product Name:3-(5-chlorothiophen-2-yl)acrylic acid
- CAS Number:69193-39-1
- Molecular formula:C7H5ClO2S
- Molecular Weight:188.6314
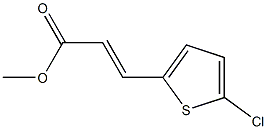
62157-63-5
3 suppliers
inquiry

69193-39-1
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium hydroxide;water in tetrahydrofuran;methanol at 0 - 20; for 2 h;
Steps:
25 EXAMPLE 25; 3-(5-Chloro-thiophen-2-yl)-(E)-acrylic acid.
EXAMPLE 25 3-(5-Chloro-thiophen-2-yl)-(E)-acrylic acid. To a mixture of 3-(5-chloro-thiophen-2-yl)-(E)-acrylic acid methyl ester (0.60 g, 2.96 mmol) in 15 ML of 1:1:1 THF/MeOH/H2O at 0° C. is added lithium hydroxide monohydrate (0.62 g, 14.7 mmol).The mixture is stirred at 0° C. for 1 h, then at room temperature for 1 h and concentrated in vacuo.The residue is diluted with EtOAc and washed with 1N HCl. The aqueous layer is extracted with EtOAc and the combined organics are washed with water (2*), dried, filtered and concentrated to provide the title compound (0.54 g, 2.86 mmol) as a white solid.The crude material can be used in the subsequent step without further purification. 1H NMR (CDCl3, 300 MHz) δ7.65 (d, 1H), 7.05 (d, 1H), 6.90 (d, 1H), 6.10 (d, 1H).
References:
US2004/102450,2004,A1 Location in patent:Page/Page column 76
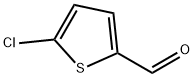
7283-96-7
272 suppliers
$8.00/5g

69193-39-1
4 suppliers
inquiry