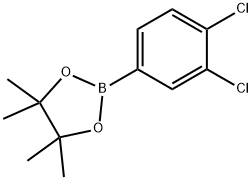
3,4-DICHLOROPHENYLBORONIC ACID, PINACOL ESTER synthesis
- Product Name:3,4-DICHLOROPHENYLBORONIC ACID, PINACOL ESTER
- CAS Number:401797-02-2
- Molecular formula:C12H15BCl2O2
- Molecular Weight:272.96
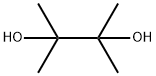
76-09-5
387 suppliers
$10.00/1g

22092-92-8
3 suppliers
inquiry

405-02-7
0 suppliers
inquiry

401797-02-2
72 suppliers
$45.00/100mg
Yield:401797-02-2 72%
Reaction Conditions:
Stage #1: diisopropopylaminoborane;1,2-dichloro-4-diazoniumbenzene tetrafluoroboratewith bis(cyclopentadienyl)titanium dichloride in acetonitrile at 20; for 2.5 h;
Stage #2: with methanol in acetonitrile at 0 - 20; for 1 h;
Stage #3: 2,3-dimethyl-2,3-butane diol in diethyl ether;acetonitrile at 20; for 4 h;Reagent/catalyst;
Steps:
33.VIA19
Example 3: General procedure D for the synthesis of the arylpinacolboronates by arylation of diisopropylaminoborane, catalysed by ferrocene (1%), followed by methanolysis and transesterification In a dried tube reactor under argon as described in example 2, the arenediazonium salt (1 mmol) and the ferrocene (ΙΟμιηοΙ, 1.8mg) were dissolved in 2mL of anhydrous CH3CN. Diisopropylaminoborane (2mmol, 226mg) was then added to the solution and the mixture was stirred for 2h30 at room temperature. The reaction mixture was quenched by a slow addition of anhydrous MeOH at 0°C (2mL) and stirred for an additional hour at room temperature. After removal of all the volatiles, 1.3eq of pinacol was added in Et20 (2mL), the mixture was stirred 4h at room temperature. The crude mixture was washed with a 50g/L CuCl2 solution (2 x 5mL). The organic layer were separated, dried over Na2S04, filtered and concentrated to dryness. The resulted oil was dissolved with CH2C12 and filtered of a pad of silica gel, eluting with CH2C12 to afford the corresponding boronate. Example 33: Synthesis of the arylpinacolboronates by arylation of diisopropylaminoborane., catalysed by a titanocene (1%), followed by methanolysis and transesterification Compounds VIAI to VIA22, IA24 and VIA26 were prepared using the procedure D (example 3), by replacing the ferrocene (1%) by the titanocene Cp2TiCl2 (1%). The yields are shown in the table 1.
References:
WO2014/9169,2014,A1 Location in patent:Page/Page column 53-54; 67-68
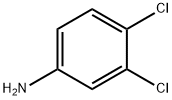
95-76-1
381 suppliers
$10.00/5g
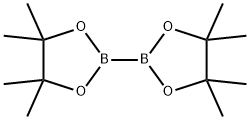
73183-34-3
590 suppliers
$6.00/5g

401797-02-2
72 suppliers
$45.00/100mg
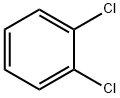
95-50-1
591 suppliers
$10.00/5g
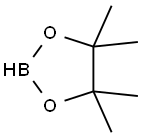
25015-63-8
222 suppliers
$22.39/5G

401797-02-2
72 suppliers
$45.00/100mg
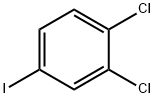
20555-91-3
193 suppliers
$8.00/5g

73183-34-3
590 suppliers
$6.00/5g

401797-02-2
72 suppliers
$45.00/100mg

95-50-1
591 suppliers
$10.00/5g

73183-34-3
590 suppliers
$6.00/5g

401797-02-2
72 suppliers
$45.00/100mg