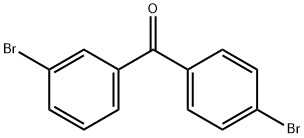
3,4'-DIBROMOBENZOPHENONE synthesis
- Product Name:3,4'-DIBROMOBENZOPHENONE
- CAS Number:83699-51-8
- Molecular formula:C13H8Br2O
- Molecular Weight:340.01
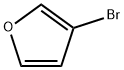
22037-28-1
345 suppliers
$24.00/5g
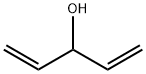
922-65-6
144 suppliers
$22.00/1g
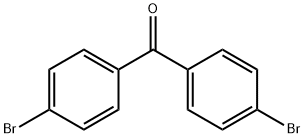
3988-03-2
215 suppliers
$6.00/1g

83699-51-8
12 suppliers
$126.00/1g
Yield:3988-03-2 30 mg ,83699-51-8 3 mg
Reaction Conditions:
Stage #1: divinylcarbinolwith 2,3-dicyano-5,6-dichloro-p-benzoquinone in dichloromethane at 25; for 18 h;Schlenk technique;Inert atmosphere;Diels-Alder Cycloaddition;
Stage #2: 3-bromofuranewith magnesium iodide in dichloromethane at 0; for 18 h;Schlenk technique;Inert atmosphere;Diels-Alder Cycloaddition;
Stage #3: with sodium methylate in methanol;dimethyl sulfoxide at 20; for 2 h;Temperature;Solvent;Reagent/catalyst;
Steps:
b1 bl) Aromatization of the double Diels-Alder adducts
In a carousel tube fitted with a PTFE septum screw cap, Diels-Alder adducts from the example a) (206 mg, 0.46 mmol), dimethyl sulfoxide (1.5 ml) and sodium methoxide solution (25 wt. % in methanol; 41 mg; 0.19 mmol) were charged. The reaction mixture was stirred at room temperature during 2 hours. 1H NMR (DMSO-d6) show the conversion of the cycloadducts and the formation of dibromobenzophenone (d 7.78, (d, J= 8.8 Hz), 7.67 (d, J=8.8Hz)). GC analysis calibrated with the commercial product show the formation of 30 mg of 4,4’-dibromobenzophenone and 3 mg of 3,4’- dibromobenzophenone, corresponding to a yield of 21 % for those 2 products.
References:
WO2019/106197,2019,A1 Location in patent:Page/Page column 12-13
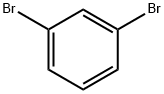
108-36-1
460 suppliers
$10.00/1g
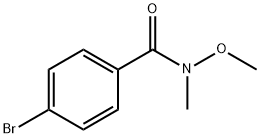
192436-83-2
82 suppliers
$19.00/1g

83699-51-8
12 suppliers
$126.00/1g
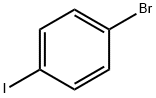
589-87-7
539 suppliers
$10.00/1g
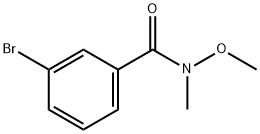
207681-67-2
49 suppliers
$156.56/5g

83699-51-8
12 suppliers
$126.00/1g
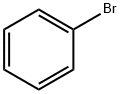
108-86-1
519 suppliers
$10.00/5g
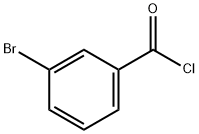
1711-09-7
209 suppliers
$9.00/5g

83699-51-8
12 suppliers
$126.00/1g
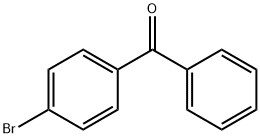
90-90-4
238 suppliers
$10.00/5g

83699-51-8
12 suppliers
$126.00/1g