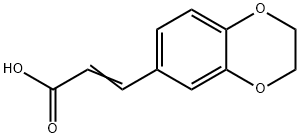
3-(2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL)ACRYLIC ACID synthesis
- Product Name:3-(2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL)ACRYLIC ACID
- CAS Number:14939-91-4
- Molecular formula:C11H10O4
- Molecular Weight:206.19
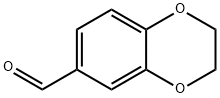
29668-44-8
281 suppliers
$6.00/1g
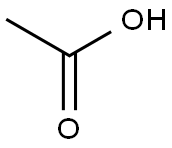
64-19-7
1564 suppliers
$10.00/25ML

14939-91-4
38 suppliers
$45.00/50mg
Yield:14939-91-4 38.9%
Reaction Conditions:
Stage #1: glacial acetic acidwith potassium carbonate at 0 - 20; for 0.0833333 h;Inert atmosphere;
Stage #2: 2,3-dihydrobenzo[b][1,4]dioxine-6-carbaldehyde at 165; for 15 h;
Steps:
18-1 18-1: Preparation of (E)-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylic acid (Compound 18b)
Anhydrous acetic acid (4.0 mL, 42.2 mmol) was slowly added at 0°C to a round-bottom flask containing potassium carbonate (1.04 g, 7.54 mmol) under argon atmosphere, and the temperature was gently raised to room temperature, followed by stirring for 5 minutes, and then 2,3-dehydrobenzo[b][1,4]dioxin-6-carbaldehyde (Compound 18a) (0.99 g, 6.03 mmol) was slowly added. The reaction mixture was stirred under reflux at 165°C for 15 hours. Upon the completion of the reaction, the temperature was lowered to room temperature (solid generating within 1 hour), and ice water was added. Thereafter, solids were filtered by using a filtration device, washed several times with water, and then dried. The obtained solid mixture was dissolved in an ethyl acetate solvent (30.0 mL), and washed twice with a saturated sodium bicarbonate aqueous solution (20.0 mL), and then the ethyl acetate layer was discarded. The sodium bicarbonate aqueous solution layer was acidified with an aqueous solution of 3N hydrogen chloride and then extracted twice with an ethyl acetate solvent. The organic layer was washed once with a saturated sodium chloride solution, dried over anhydrous magnesium sulfate, evaporated under reduced pressure, and then sufficiently dried, to give (E)-3-(2,3-dehydrobenzo[b][1,4]dioxin-6-yl)acrylic acid (Compound 18b) (0.48 g, 38.9 %) as a white solid.1H NMR (400 MHz, CDCl3) : δ 7.67 (1H, d, J = 16.0 Hz), 7.08 (1H, d, J = 2.0 Hz), 7.06 (1H, dd, J = 8.4, 2.0 Hz), 6.88 (1H, d, J = 8.4 Hz), 6.29 (1H, d, J = 15.6 Hz), 4.31-4.26 (4H, m).
References:
EP4023301,2022,A2 Location in patent:Paragraph 0135-0137
141-82-2
757 suppliers
$5.00/25g

29668-44-8
281 suppliers
$6.00/1g

14939-91-4
38 suppliers
$45.00/50mg
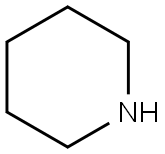
110-89-4
3 suppliers
$15.96/100ML

29668-44-8
281 suppliers
$6.00/1g

14939-91-4
38 suppliers
$45.00/50mg
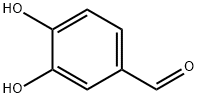
139-85-5
826 suppliers
$5.00/1g

14939-91-4
38 suppliers
$45.00/50mg
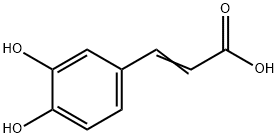
331-39-5
700 suppliers
$5.00/100mg

14939-91-4
38 suppliers
$45.00/50mg