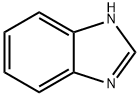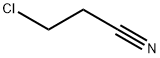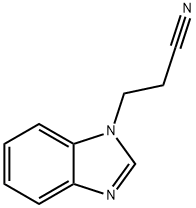
3-(1H-Benzimidazol-1-yl)propanenitrile synthesis
- Product Name:3-(1H-Benzimidazol-1-yl)propanenitrile
- CAS Number:4414-84-0
- Molecular formula:C10H9N3
- Molecular Weight:171.2
Yield:4414-84-0 96.8%
Reaction Conditions:
in neat (no solvent, gas phase) at 40; for 2 h;Microwave irradiation;Molecular sieve;Green chemistry;Michael Addition;
Steps:
2.1 General procedure for one-pot microwave-assisted synthesis of acrylonitrile adducts
General procedure: A mixture of primary aliphatic and aromatic amines (1a-k) (1mmol) as Michael donors and acrylonitrile (2) (2.5mmol) as Michael acceptor were performed as one-pot microwave-assisted synthesis at 40°C for 2h. The reaction was catalyzed by molecular sieves 4? (0.12g). The formation of the products was monitored at different intervals of time using thin layer chromatographic (TLC) technique. Constantly monitoring with TLC inferred that the reaction completes within 2h of time. TLC confirms the formation of the desired product where the reactants n-propylamine (1a), acrylonitrile (2), co-spot (CO) and the expected product n-propyliminobis-propionitrile (3a) were examined as a preliminary investigation. Prominent conversion of mono or diadduct was observed from the TLC. It showed highly intense single spot with negligible unreacted substrates.
References:
Das, Parineeta;Devi, Nirmala;Puzari, Amrit [Journal of the Indian Chemical Society,2022,vol. 99,# 5,art. no. 100411]