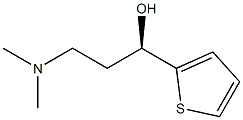
(2S)-HYDROXY(PHENYL)ACETIC ACID (1S)-3-(DIMETHYLAMINO)-1-(2-THIENYL)PROPAN-1-OL (1:1) (SALT) synthesis
- Product Name:(2S)-HYDROXY(PHENYL)ACETIC ACID (1S)-3-(DIMETHYLAMINO)-1-(2-THIENYL)PROPAN-1-OL (1:1) (SALT)
- CAS Number:287737-72-8
- Molecular formula:C17H23NO4S
- Molecular Weight:337.4338
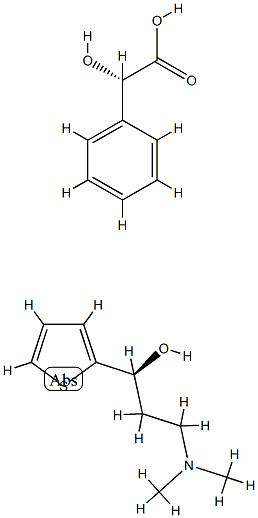
17199-29-0
585 suppliers
$5.00/10g

287737-72-8
1 suppliers
inquiry
Yield:-
Reaction Conditions:
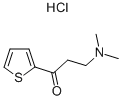
Stage #1: 3-(dimethylamino)-1-(thiophen-2-yl)propan-1-onewith sodium hydroxide;sodium tetrahydroborate in methanol;water at 0 - 20; pH=10;
Stage #2: with hydrogenchloride in water; pH=1.5; for 0.333333 h;
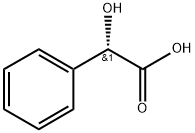
Stage #3: (S)-Mandelic acidwith sodium hydroxideProduct distribution / selectivity;more than 3 stages;
Steps:
9
A solution of 90 g of AT-ONE, in 290 ml methanol and 145 ml of water was cooled to OC and 14 ml of NaOH [47%] were gradually added till pH 10. To the resulting solution was added portion added 12.1 g of sodium borohydride and the mixture was allowed to warm to room temperature overnight. The methanol was evaporated under reduced pressure and 250 ml were added followed by the slowly addition of concentrated HCl till pH 1.5 and stirred for an additional 20 minutes. After basification with NaOH the phases were separated, the water phase was washed with MTBE and the combined organic phases were washed with brine. To the MTBE solution was added a solution of 16.4 g of (S)-mandelic acid in 40 ml ethanol and the resulting mixture was stirred at reflux for 1.25 hours and then cooled to room temperature. The resulting solid was filtered from the mother liquor, washed with MTBE and dried in a vacuum oven to give 25 g of (S)-AT-OL mandelate.
References:
US2008/15363,2008,A1 Location in patent:Page/Page column 6
5424-47-5
215 suppliers
$9.00/5g

287737-72-8
1 suppliers
inquiry
132335-49-0
35 suppliers
inquiry

287737-72-8
1 suppliers
inquiry
88-15-3
606 suppliers
$5.00/10g

287737-72-8
1 suppliers
inquiry