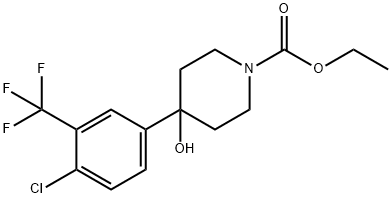
Ethyl 4-(4-chloro-3-(trifluoromethyl)phenyl)-4-hydroxypiperidine-1-carboxylate synthesis
- Product Name:Ethyl 4-(4-chloro-3-(trifluoromethyl)phenyl)-4-hydroxypiperidine-1-carboxylate
- CAS Number:21928-40-5
- Molecular formula:C15H17ClF3NO3
- Molecular Weight:351.75
445-01-2
237 suppliers
$13.00/25g

106-93-4
395 suppliers
$15.00/5g
29976-53-2
382 suppliers
$5.00/5g

21928-40-5
23 suppliers
inquiry
Yield:21928-40-5 85.7%
Reaction Conditions:
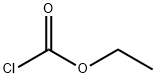
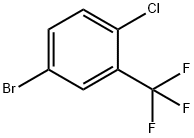
Stage #1: 5-bromo-2-chlorobenzotrifluoridewith magnesium;calcium chloride in diethyl ether at 20;Autoclave;
Stage #2: ethylene dibromidewith iodine in diethyl ether; for 1 h;Reflux;
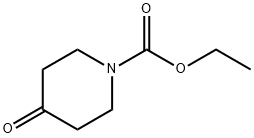
Stage #3: N-ethoxycarbonyl-4-piperidone in diethyl ether; for 1.5 h;
Steps:
1.5 Preparation of 1-ethoxycarbonyl-4- (3-trifluoromethyl-4-chlorophenyl) -4-piperidinol (G) (abbreviated as carbonylpiperidinol)
In an absolutely anhydrous (1: 1) autoclave equipped with a mechanical stirrer, a condenser, a thermometer, a dropping funnel and an anhydrous calcium chloride drying tube500ml three-mouth reaction bottle, at room temperature by adding 2.5g (0.103mol) of magnesium metal and 20ml anhydrous ether, slowly start stirringmix.27 g (0.104 mol) of 2-chloro-5-bromo-trifluorotoluene (referred to as bromide) was dissolved in 130 ml of anhydrous ether, Stirring uniformly to obtain the material liquid (W);15 ml of the material solution (W) was added to the above reactants, and then 0.13 g of iodine and 0.2 g of 1,2-dibromoethane were added,Hair dye reaction, to be iodine disappeared, the reaction is slightly slow, slowly dropping material (W). The addition was continued and reflux was continued for one hour. reactionAfter cooling to room temperature, a solution of carbonyl piperidone (F) [carbonyl piperidone 13.6 g(0.0795 mol) + 40 ml of anhydrous ether] was added dropwise, and the reaction was stirred for 1.5 hours. A 20% by weight ammonium chloride solution was addedAnd the mixture was heated to reflux for 15 minutes and allowed to stand at room temperature for 30 minutes. The aqueous layer (lower layer) was released, and the residual liquid (upper layer) was heated to an external temperature of 55 ° C atmospheric pressure distillation recovery ether, hot discharge, frozen overnight, precipitation of solids. Filtration, a small amount of washed 1, drained, dry productionProduct (G) 24.1 g, yield 85.7%, m.p. 118-126 ° C.
References:
CN106187863,2016,A Location in patent:Paragraph 0144; 0145; 0146; 0147; 0148
![4-[4-Chloro-3-(trifluoromethyl)phenyl]-4-piperidinol](/CAS/GIF/21928-50-7.gif)