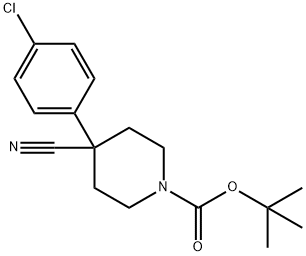
TERT-BUTYL 4-(4-CHLOROPHENYL)-4-CYANOPIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(4-CHLOROPHENYL)-4-CYANOPIPERIDINE-1-CARBOXYLATE
- CAS Number:218451-34-4
- Molecular formula:C17H21ClN2O2
- Molecular Weight:320.81
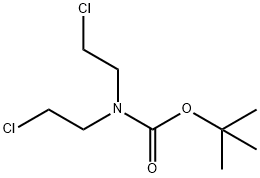
118753-70-1
176 suppliers
$6.00/1g
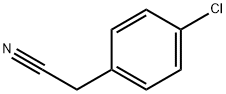
140-53-4
485 suppliers
$13.00/25g

218451-34-4
31 suppliers
inquiry
Yield:218451-34-4 71%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 60;Inert atmosphere;
Steps:
1d.1
Step 1. To the solution of 2-(4-chlorophenyl)acetonitrile (20.1 g, 132.59 mmol, 1.00 equiv) and tert-butyl N,N-bis(2-chloroethyl)carbamate (35.4 g, 146.19 mmol, 1.10 equiv) in anhydrous DMF (200 mL) was added sodium hydride (27g, 60% in mineral oil,666.73 mmol, 3.00 equiv) portionwise at 0°C under N2 over 2 hr. The resulting solution was stirred at 60°C for 1.5 hr, and then stirred overnight at room temperature. The reaction was quenched by the careful addition of 250 mL of sat. aq. NH4C1. The resulting solution was extracted with 3x200 mL of dichloromethane. The combined extracts were washed with 2x300 mL of brine, then dried over anhydrous sodium sulfate andconcentrated under vacuum. The residue was purified on a silica gel column with dichloromethane/petroleum ether (1:1). 30 g (71%) of tert-butyl 4-(4-chlorophenyl)-4- cyanopiperidine-1-carboxylate was obtained as a yellow solid. TLC: Rf = 0.15; ethyl acetate/petroleum ether = 1/5.
References:
WO2015/32286,2015,A1 Location in patent:Page/Page column 310; 311
623-03-0
460 suppliers
$6.00/25g

118753-70-1
176 suppliers
$6.00/1g

218451-34-4
31 suppliers
inquiry
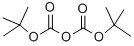
24424-99-5
839 suppliers
$13.50/25G

218451-34-4
31 suppliers
inquiry

118753-70-1
176 suppliers
$6.00/1g

218451-34-4
31 suppliers
inquiry