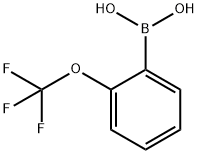
2-(Trifluormethoxy)phenylboronic acid synthesis
- Product Name:2-(Trifluormethoxy)phenylboronic acid
- CAS Number:175676-65-0
- Molecular formula:C7H6BF3O3
- Molecular Weight:205.93
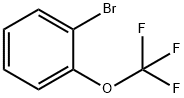
64115-88-4
238 suppliers
$15.00/5g

175676-65-0
216 suppliers
$6.00/1g
Yield: 65%
Reaction Conditions:
Stage #1:1-bromo-2-(trifluoromethoxy)benzene with n-butyllithium in tetrahydrofuran at -78; for 0.75 h;
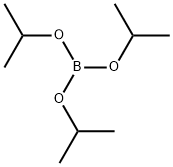
Stage #2: with Triisopropyl borate in tetrahydrofuran at -78 - 20; for 16 h;
Stage #3: with hydrogenchloride;sodium hydroxide;watermore than 3 stages;
Steps:
1.3 EXAMPLE 1; 20 5-METHYL-L-R2&No.x0;-(TRIFLUOROMETHOXY)-L O L&No.x0;-BIPHENYL-3-YLL-LH-PYRAZOLE-3- carboxamide; Step 3: 2-(TRIFLUOROMETHOXY) PHENYLBORONIC ACID
n-Butyllithium (5.9 ml, 9.5 mmol) was added to a solution of 1-BROM-2- (trifluoromethoxy) benzene (2 g, 8.2 mmol) in tetrahydrofuran (28 ML) at-78°C and stirred for 45 minutes. Triisopropyl borate (2. 58 ml, 11.1 mmol) was added dropwise to the reaction mixture and the solution was slowly brought to room temperature over 16 hours. The reaction mixture was quenched with water, made basic with 2N NAOH and extracted with ethyl acetate. The aqueous solution was acidified with 2N HCI, stirred for 1 hour at room temperature and extracted into ethyl acetate. The organic layer was washed with water, brine solution and dried over sodium sulfate. It was filtered and concentrated to give the product (1.10 g, 65%) as a white solid. &No.x0;HNMR (CDCL3) ( 8, ppm): 7.96 (dd, J= 7.2, 1.6 Hz, 1 H), 7.53 (ddd, J = 9.1, 7.3, 1. 8 HZ, 1 H), 7.38 (td, J = 7.3, 0.7 Hz, 1 H), 7.28 (d, J = 8.2 Hz, 1 H), 5.25 (br s, 2H). MS: m/e 206.9 (M+1) +.
References:
MERCK & CO., INC. WO2004/92140, 2004, A1 Location in patent:Page 38
5419-55-6
385 suppliers
$12.00/5g

64115-88-4
238 suppliers
$15.00/5g

175676-65-0
216 suppliers
$6.00/1g

5419-55-6
385 suppliers
$12.00/5g

175676-65-0
216 suppliers
$6.00/1g
456-55-3
305 suppliers
$6.00/10g

175676-65-0
216 suppliers
$6.00/1g