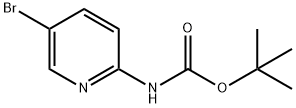
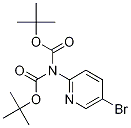
2-(N-BOC-AMINO)-5-BROMOPYRIDINE synthesis
- Product Name:2-(N-BOC-AMINO)-5-BROMOPYRIDINE
- CAS Number:159451-66-8
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13
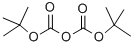
24424-99-5
833 suppliers
$13.50/25G

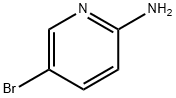
159451-66-8
145 suppliers
$12.00/1g
Yield: 85%
Reaction Conditions:
Stage #1:5-bromo-2-pyridylamine with sodium hexamethyldisilazane in tetrahydrofuran at 0; for 0.0833333 h;
Stage #2:di-tert-butyl dicarbonate in tetrahydrofuran at 20; for 0.5 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water; pH=7 - 8
Steps:
1.B 1B. Preparation of tert-Butyl 5-bromopyridin-2-ylcarbamate
To a solution of 2-amino-5-bromopyridine (8.65 g, 50 mmol) in THF (100 mL) at 0° C. was added sodium bis(trimethylsilyl)amide (1 M solution in THF, 105 mL, 105 mmol). After addition, the reaction was stirred at 0° C. for 5 min, and then di-tert-butyl dicarbonate (12 g, 55 mmol) was added in several portions. After addition, the reaction was stirred at RT for 30 min, and then diluted with water (60 mL) and neutralized by adding ice-cold 1N HCl (about 90 mL) to pH=78. The resultant mixture was extracted with EtOAc (3×100 mL). The combined organic layers were washed with saturated NaCl. The organic layer was dried (Na2SO4), filtered and concentrated. The resulting residue was purified by silica gel (330 g) column chromatography eluting with a gradient of EtOAc (0-50%) in hexane to give the title compound as an off-white solid (11.6 g, 85%). LC/MS (method A): retention time=3.47 min, (M+H-tBu))+=217.
References:
Sun, Chongqing;Ewing, William R.;Sulsky, Richard B.;Huang, Yanting US2006/155126, 2006, A1 Location in patent:Page/Page column 8
1072-97-5
689 suppliers
$9.00/1g

24424-99-5
833 suppliers
$13.50/25G

159451-66-8
145 suppliers
$12.00/1g

1072-97-5
689 suppliers
$9.00/1g

24424-99-5
833 suppliers
$13.50/25G
209959-28-4
26 suppliers
$89.00/500mg

159451-66-8
145 suppliers
$12.00/1g

209959-28-4
26 suppliers
$89.00/500mg

159451-66-8
145 suppliers
$12.00/1g

1072-97-5
689 suppliers
$9.00/1g

159451-66-8
145 suppliers
$12.00/1g