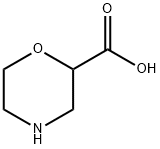
2-MORPHOLINECARBOXYLIC ACID HCL synthesis
- Product Name:2-MORPHOLINECARBOXYLIC ACID HCL
- CAS Number:300582-83-6
- Molecular formula:C5H9NO3
- Molecular Weight:131.13
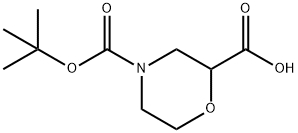
189321-66-2
144 suppliers
$5.00/250mg

300582-83-6
85 suppliers
$90.00/100mg
Yield:-
Reaction Conditions:
with trifluoroacetic acid in dichloromethane for 1 h;
Steps:
28
Example 28: 4-(2-Hydroxy-2-(4-(5-(3-phenyl-4-(trifluoromethyl)isoxazol-5-yl)-l,2,4- oxadiazol-3-yl)phenyl)ethyl)morpholine-2-carboxylic acid[00278] 4-(tert-Butoxycarbonyl)morpholine-2-carboxylic acid (69.5 mg, 0.301 mmol) was treated with TFA/DCM for 1 hour. Solvents were removed in vacuo and the resulting material was dried. The solids were dissolved in 2-propanol (2 mL) and DMSO (1 mL). 3-(4-(oxiran-2-yl)phenyl)-5-(3-phenyl-4-(trifluoromethyl)isoxazol-5-yl)- 1,2,4- oxadiazole (30 mg, 0.075 mmol) and cesium carbonate (98 mg, 0.301 mmol) were added. The reaction mixture was heated at 80 0C overnight. LCMS indicated almost complete conversion to desired product and no starting material. The reaction mixture was filtered and purified by HPLC. HPLC conditions: PHENOMENEX Luna C18 5 micron column (250 x 30mm); 25-100% CH3CN/water (0.1% TFA); 25 minute gradient; 30 mL/min. Isolated fractions with correct mass were freeze-dried overnight to yield 21 mg of 4-(2-hydroxy-2-(4-(5-(3-phenyl-4-(trifluoromethyl)isoxazol-5-yl)-l,2,4-oxadiazol-3- yl)phenyl)ethyl) morpholine-2-carboxylic acid as a TFA salt. 1H NMR (400 MHz, MeOH-d3) δ ppm 8.23 (2 H, d, J=I .91 Hz), 7.69 (4 H, dd, J=12.08, 7.69 Hz), 7.54-7.65 (3 H, m), 5.29 (1 H, td, J=6.81, 4.39 Hz), 4.41-4.65 (1 H, m), 4.08-4.37 (1 H, m), 3.92- 4.05 (2 H, m), 3.56-3.91 (1 H, m), 3.44 (3 H, t, J=6.81 Hz), 3.12-3.38 (1 H, m). MS (m+1) = 531. HPLC Peak RT = 3.24 minutes (Analytical Method A). Purity = 85%.
References:
BRISTOL-MYERS SQUIBB COMPANY;GILMORE, John L.;SHEPPECK, James E. WO2011/17578, 2011, A1 Location in patent:Page/Page column 134