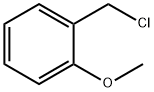
2-Methoxybenzyl chloride synthesis
- Product Name:2-Methoxybenzyl chloride
- CAS Number:7035-02-1
- Molecular formula:C8H9ClO
- Molecular Weight:156.61
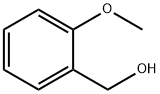
612-16-8
224 suppliers
$11.00/5g

7035-02-1
113 suppliers
$59.00/10 g
Yield:7035-02-1 96%
Reaction Conditions:
with thionyl chloride;triethylamine in dichloromethane at 20; for 1 h;
Steps:
64 (2-methoxyphenyl)methyl 4-(4-chlorophenyl)-1 -(2-methoxyethyl)-6-methyl- 2-oxo-3,4-dihydropyridine-5-carboxylate
2-methoxybenzyl alcohol (268 μ Ι_, 2.0 mmol) and triethylamine (335 μ Ι_, 2.4 mmol) were dissolved in anh. DCM (7 ml_). Thionyl chloride (218 μΙ_, 3.0 mmol) was added slowly. The reaction mixture was stirred at RT for 1 h. The reaction mixture was washed with an aqueous solution of HCI 1 N. The organic phase was dried over MgS04. The solvent was removed under reduced pressure to give the desired 2-methoxybenzyl chloride (300 mg, 96 %) as a yellowish oil . 4-(4-chlorophenyl)-1 -(2-methoxyethyl)-6-methyl-2-oxo-3,4-dihydropyridine-5-carboxylic acid (example 74, 85 mg, 0.26 mmol) was dissolved in anhydrous DM F (1 m L) then cesium carbonate (169 mg, 0.52 mmol) and 2-methoxybenzyl chloride (81 mg, 0.52 mmol) were added. The reaction mixture was stirred at RT for 18 h. The solvent was removed under reduced pressure. The residue was dissolved in EtOAc and water. The aqueous phase was extracted with EtOAc. The combined organic layers were washed with brine and dried over MgS04. The solvent was removed under reduced pressure. Purification of the crude by flash chromatography using a mixture of Cyclohexane/EtOAc (8/2) as eluent gave the desired (2- meth oxyphenyl )methyl 4-(4-chlorophenyl)-1-(2-methoxyethyl)-6-methyl-2-oxo-3,4- dihydropyridine-5-carboxylate as a yellowish oil (36 mg, 31 %). 1H NMR (300 MHz, CDCI3) δ 2.60 (s, 3H), 2.71 (dd, J = 15.9, 2.4 Hz, 1 H), 2.91 (dd, J = 15.9, 7.6 Hz, 1 H), 3.31 (s, 3H), 3.36 (ddd, J = 9.8, 8.3, 3.7 Hz, 1H), 3.46 (dt, J = 9.8, 4.2 Hz, 1H), 3.74 (s, 3H), 3.69-3.78 (m, 1H), 4.13-4.21 (m, 2H), 5.10 (d, J = 12.9 Hz, 1H), 5.19 (d, J= 12.9 Hz, 1H), 6.80-6.85 (m, 2H), 6.98 (dd, J = 7.8, 1.6 Hz, 1H), 7.09 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 7.26 (t, J = 7.8 Hz,1H). 13C NMR (75 MHz, CDCI3) δ 17.0, 36.8, 38.6, 41.8, 55.1, 58.8, 61.8, 71.0, 110.1, 110.3, 120.2, 124.2, 128.4, 128.6, 129.1, 129.2, 132.4, 139.8, 150.6, 157.2, 167.1, 169.0. MS [M+H]+ 444. HRMS : calcd for C24H27N05CI, [M+H]+ 444.1578, found 444.1585.
References:
UNIVERSITE DE LILLE 2 DROIT ET SANTE;INSTITUT PASTEUR DE LILLE;INSERM (INSTITUT NATIONAL DE LA SANTé ET DE LA RECHERCHE MéDICALE);CHARTON, Julie;DEPREZ, Benoit;LEROUX, Florence;STAELS, Bart;MUHR-TAILLEUX, Anne;HENNUYER, Nathalie;LESTAVEL, Sophie;PICON, Sylvain;AKNIN, Karen;BOULAHJAR, Rajaa;DUBANCHET, Barbara WO2016/16238, 2016, A1 Location in patent:Page/Page column 105-106
50-00-0
895 suppliers
$10.00/25g
100-66-3
538 suppliers
$10.00/5G

7035-02-1
113 suppliers
$59.00/10 g
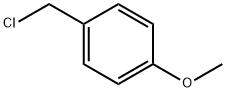
824-94-2
454 suppliers
$24.19/5 g
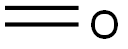
50-00-0
895 suppliers
$10.00/25g

100-66-3
538 suppliers
$10.00/5G

7035-02-1
113 suppliers
$59.00/10 g
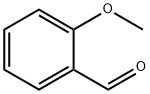
135-02-4
405 suppliers
$10.00/10g

7035-02-1
113 suppliers
$59.00/10 g
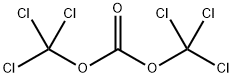
32315-10-9
432 suppliers
$10.00/1g

612-16-8
224 suppliers
$11.00/5g

7035-02-1
113 suppliers
$59.00/10 g