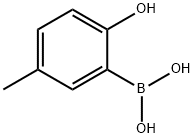
2-HYDROXY-5-METHYLPHENYLBORONIC ACID synthesis
- Product Name:2-HYDROXY-5-METHYLPHENYLBORONIC ACID
- CAS Number:259209-21-7
- Molecular formula:C7H9BO3
- Molecular Weight:151.96
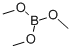
121-43-7
359 suppliers
$13.00/25mL
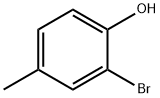
6627-55-0
255 suppliers
$6.00/5g

259209-21-7
65 suppliers
$23.00/100mg
Yield:259209-21-7 60%
Reaction Conditions:
Stage #1:2-bromo-p-cresol with n-butyllithium in diethyl ether;hexane at 20; for 2 h;Cooling;Inert atmosphere;
Stage #2:Trimethyl borate in diethyl ether;hexane at 20; for 15.5 h;Cooling;Inert atmosphere;
Stage #3: with hydrogenchloride in diethyl ether;hexane;water for 0.5 h;Cooling with ice;
Steps:
Synthesis of boronic acid derivatives
General procedure: A solution of n-butyllithium (1.7 M in hexane, 38 mL ) was slowly added to a cooled (-80 °C) solution of 30 mmol 2-bromo-4-alkylphenol or 30 mmol of 2-bromo-1-methoxy-4-alkylphenol respectively, in dry ether (80 mL). The mixture was then allowed to warm up and stirred at rt for 2 h under an argon atmosphere. It was then cooled again (-80 °C) and trimethyl borate (5.58 mL, 50 mmol) was rapidly added. The mixture was stirred at -80 °C for 0.5 h and then at rt for 15 h under an argon atmosphere. Then 20 mL of 2 M aq. HCl solution were added slowly into the ice-cold reaction mixture and the mixture was stirred again for 0.5 h, while the milky white emulsion gradually became clear. The ethereal layer was then separated and the aqueous layer was extracted with diethyl ether (3 times with 100 mL each). The combined ether solutions were dried (MgSO4) and after filtration the solvent was evaporated under reduced pressure. The residual solid was recrystallized from hot diethyl ether : toluene, 3:7) to give a white solid.
References:
Fuchs, Alexander;Baur, Roland;Schoeder, Clara;Sigel, Erwin;Müller, Christa E. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 24,p. 6908 - 6917] Location in patent:supporting information
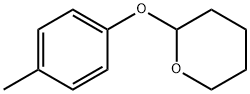
13481-09-9
15 suppliers
inquiry

259209-21-7
65 suppliers
$23.00/100mg
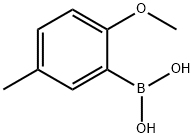
127972-00-3
168 suppliers
$15.00/5mg

259209-21-7
65 suppliers
$23.00/100mg
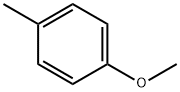
104-93-8
397 suppliers
$6.00/25g

259209-21-7
65 suppliers
$23.00/100mg
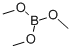
121-43-7
359 suppliers
$13.00/25mL
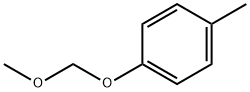
25458-49-5
13 suppliers
inquiry

7732-18-5
476 suppliers
$12.69/100ml

259209-21-7
65 suppliers
$23.00/100mg