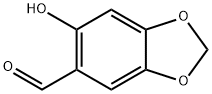
2-Hydroxy-4,5-methylenedioxybenzaldehyde synthesis
- Product Name:2-Hydroxy-4,5-methylenedioxybenzaldehyde
- CAS Number:4720-68-7
- Molecular formula:C8H6O4
- Molecular Weight:166.13
Yield:4720-68-7 93.2%
Reaction Conditions:
with tin(IV) chloride in dichloromethane at 0; for 2 h;Friedel-Crafts type formylation;
Steps:
4.2.3. 6-Hydroxybenzo[1,3]dioxole-5-carboxaldehyde (10)
In a two-neck flask, 40 g of 9 (0.222 mol) were dissolved in 500 mL CH2Cl2 and the solution cooled to 0 °C. Through a dropping funnel, 52 mL (0.444 mol) of SnCl4 were added dropwise, followed by slow addition of 22 mL (0.244 mol) of Cl2CHOCH3, causing the formation of a precipitate. The reaction was stirred for 2 h and was then poured over ice. The mixture was partitioned and the organic layer was washed with of 2M HCl (3 × 20 mL), and then with water (2 × 50 mL). The organic solution was dried over Na2SO4, filtered, and the solvent removed by rotary evaporation to afford a solid, which was triturated under cold MeOH, filtered, and dried to yield 34.38 g (93.2%) of 10 as a tan solid: mp 120-121 °C (Lit [28]. mp 125-126). 1H NMR (CDCl3): δ 9.63 (s, 1H, CHO), 6.87 (s, 1H, ArH), 6.47 (s, 1, ArH), 6.02 (s, 2H, ArOCH2O), 1.54 (s, 1H, ArOH). CIMS: m/z 167 (M + H+, 100). Anal. (C8H6O4) C, H.
References:
Cueva, Juan Pablo;Chemel, Benjamin R.;Juncosa Jr., Jose I.;Lill, Markus A.;Watts, Val J.;Nichols, David E. [European Journal of Medicinal Chemistry,2012,vol. 48,p. 97 - 107] Location in patent:experimental part
50-00-0
886 suppliers
$10.00/25g
533-31-3
618 suppliers
$5.00/100mg

4720-68-7
34 suppliers
$45.00/10mg

533-31-3
618 suppliers
$5.00/100mg
122-51-0
504 suppliers
$10.00/5ml

4720-68-7
34 suppliers
$45.00/10mg

533-31-3
618 suppliers
$5.00/100mg
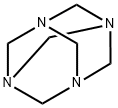
100-97-0
0 suppliers
$14.00/250G

4720-68-7
34 suppliers
$45.00/10mg

533-31-3
618 suppliers
$5.00/100mg
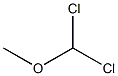
4885-02-3
191 suppliers
$10.00/1g

4720-68-7
34 suppliers
$45.00/10mg
