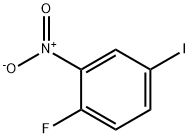
2-Fluoro-5-iodonitrobenzene synthesis
- Product Name:2-Fluoro-5-iodonitrobenzene
- CAS Number:364-75-0
- Molecular formula:C6H3FINO2
- Molecular Weight:267
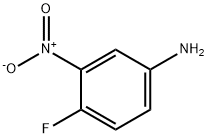
364-76-1
286 suppliers
$7.00/10g

364-75-0
101 suppliers
$5.00/250mg
Yield: 14%
Reaction Conditions:
with diiodomethane;isopentyl nitrite at 20 - 70; for 2 h;
Steps:
46
Add isoamyl nitrite (18. 6 g, 160 mmol) to a suspension of 4-fluoro-3- nitrophenylamine (5. 0 g, 32 mmol) in diiodomethane (150 mL). Stir under nitrogen at room temperature for 1 h. Heat at 70 °C for 1 h. Cool to room temperature and concentrate. Dilute with dichloromethane (500 mL) and water (100 mL). Collect the organic, concentrate and purify (silica gel chromatography, eluting with a gradient of 100 : 0 to 80 : 20 hexanes : ethyl acetate), to give the title compound as a yellow oil (1. 22 g, 14%). 1H NMR (300 MHz, CDC13) 6 7. 02-7. 11 (m, 1H), 7. 89-7. 97 (m, 1H), 8. 32-8. 39 (dd, J = 2. 2 Hz, 6. 9 Hz, 1H).
References:
ELI LILLY AND COMPANY WO2005/94822, 2005, A1 Location in patent:Page/Page column 46
1493-27-2
512 suppliers
$5.00/10g

364-75-0
101 suppliers
$5.00/250mg
352-34-1
369 suppliers
$7.00/5g

364-75-0
101 suppliers
$5.00/250mg

364-76-1
286 suppliers
$7.00/10g
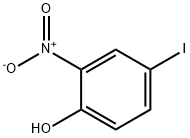
21784-73-6
51 suppliers
$75.00/500mg

364-75-0
101 suppliers
$5.00/250mg