
2-CHLORO-N-(5-CHLORO-2-METHOXYPHENYL)ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(5-CHLORO-2-METHOXYPHENYL)ACETAMIDE
- CAS Number:35588-41-1
- Molecular formula:C9H9Cl2NO2
- Molecular Weight:234.08
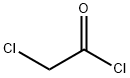
79-04-9
373 suppliers
$12.00/5g
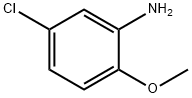
95-03-4
121 suppliers
$13.00/25g

35588-41-1
18 suppliers
$28.60/50MG
Yield:35588-41-1 81%
Reaction Conditions:
Stage #1: 5-chloro-2-methoxyanilinewith triethylamine in dichloromethane at 20; for 0.0833333 h;
Stage #2: chloroacetyl chloride in dichloromethane at 20;
Steps:
2-Chloro-N-(5-chloro-2-methoxyphenyl)acetamide (67).
Triethylamine (265 μL, 1.90 mmol,3.0 eq) was added to a solution of 5-chloro-2-methoxyaniline (100 mg, 0.635 mmol, 1.0 eq) indichloromethane (3 mL), and the reaction mixture was stirred at room temperature for 5 min.Chloroacetyl chloride (76 μL, 0.954 mmol, 1.5 eq) was added to the mixture, and stirringcontinued overnight. Water (10 mL) was added, and the mixture was extracted withdichloromethane (2x). The combined organics were dried over Na2SO4, filtered, andconcentrated in vacuo. The residue was purified by flash chromatography on silica gel to 120 mg (81%) of the title compound as an off-white solid. 1H NMR (300 MHz, CDCl3) δ 8.93 (s,1H), 8.41 (d, J = 2.6 Hz, 1H), 7.06 (dd, J = 8.7, 2.5 Hz, 1H), 6.82 (d, J = 8.7 Hz, 1H), 4.20 (s,1H), 3.91 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.71, 146.85, 127.45, 126.11, 124.14, 119.60,110.87, 56.17, 43.05.
References:
Qunies, Alshaima'a M.;Mishra, Nigam M.;Spitznagel, Brittany D.;Du, Yu;Acu?a, Valerie S.;David Weaver;Emmitte, Kyle A. [Bioorganic and Medicinal Chemistry Letters,2022,vol. 76,art. no. 129013] Location in patent:supporting information